38th International Symposium on Intensive Care and Emergency Medicine
2018, Critical Care
https://doi.org/10.1186/S13054-018-1973-5…
215 pages
1 file

Sign up for access to the world's latest research
Abstract
Introduction: Conventional assay technique to quantify vascular permeability in animal studies requires sacrifice animals; this becomes a barrier to evaluate of temporal changes or responses to therapeutic approaches in a single individual. In vivo fluorescence imaging potentially quantifies vascular permeability without sacrifice animals. However, the use of this noninvasive approach for the assessment of vascular permeability in remote organ injury caused by systemic inflammatory disease such as sepsis has not been reported. Methods: Cecal ligation and puncture (CLP)-induced septic mouse model was compared to sham and hydrocortisone pretreated (CLP + HC) mouse models. The lung was assumed as an injured remote organ and the footpad was assumed as a noninvasive observational site. The mixture of Evans blue (EB) and fluorescent dye of Genhance 750 were injected into mice, and the extraction of EB in harvested lung was assessed as a conventional indicator of vascular permeability. Fluorescent intensities in the harvested lung or footpad were assessed and their correlation was analyzed to investigate this novel, noninvasive approach to estimation of lung vascular permeability. Results: EB extraction in the harvested lung in the CLP group was significantly higher than in the other groups (CLP vs. sham, P=0.0012; CLP vs. CLP + HC, P=0.011). Fluorescent intensity in the footpad and harvested lung in the CLP group was also significantly higher than in the other groups (footpad, CLP vs. sham, P<0.0001; CLP vs. CLP + HC, P=0.0004; lung, CLP vs. sham, P<0.0001; CLP vs. CLP + HC, P<0.0001). The fluorescent intensity of the footpad was strongly correlated with that of the lung (r=0.95). Conclusions: The fluorescence imaging technique may be useful for assessment of vascular permeability based on EB quantification. The footpad fluorescent intensity was strongly correlated with that of the lung, and may be a suitable indicator in noninvasive estimation of lung vascular permeability.
Figures (380)




































![Conclusions: In clAls an appropriate empirical antibiotic therapy and an early infection source control are closely associated with better outcomes. score at infection which was higher in IAAT (p=0.04). Secondary peri- tonitis was the main type of clAl (45.5% in IIAT and 40.9% in IAAT) followed by abdominal abscess and biliary tract infection. Secondary bacteraemia was significantly higher in IIAT (p=0.03). Conversely, IAAT had an higher rate of adequate source control (p=0.01). Empi- rical therapy of IAAT patients included more frequently anti gram- positive (p=0.016) and carbapenems (p=0.01), while empirical dual anti gram negative and antifungal coverages rate were not different (Fig. 1). MDR and polimicrobial infections rate was significantly higher in II|AT when associated with septic shock at occurrence of infection (p=0.03; Fig. 2). IAAT showed significantly lower mortality at 28 and 90 days (p<0.01) as well as higher rate of clinical cure and microbio- logical eradication than IIAT (p<0.01). At the multivariate analysis, adequate source control [p=0.04, OR 0.25 (0.09-0.65)] and IIAT [(p<0.01, OR 11.4 (4.02-32.3)] turned out to be independently related with 28 days mortality. P065](https://figures.academia-assets.com/98026244/figure_029.jpg)






![Table 1 (abstract PO78). Bacterial count in lung, spleen and blood at euthanasia. Median [IQR]](https://figures.academia-assets.com/98026244/table_013.jpg)





























![Fig. 1 (abstract P126). Ninety-day mortality among patients in the LEVO-CTS trial [3] in the subgroup of isolated CABG patients (n=563)](https://figures.academia-assets.com/98026244/figure_052.jpg)




















































































































































![[1] Joosten et al. Anesthesiology 128:55-66, 2018](https://figures.academia-assets.com/98026244/figure_168.jpg)











































































































































Related papers
American Journal of Physiology-Lung Cellular and Molecular Physiology, 2021
To develop a dynamic in vivo near-infrared (NIR) fluorescence imaging assay to quantify sequential changes in lung vascular permeability-surface area product (PS) in rodents. Dynamic NIR imaging methods for determining lung vascular permeability-surface area product were developed and tested on non-irradiated and 13 Gy irradiated rats with/without treatment with lisinopril, a radiation mitigator. A physiologically-based pharmacokinetic (PBPK) model of indocyanine green (ICG) pulmonary disposition was applied to in vivo imaging data and PS was estimated. In vivo results were validated by five accepted assays: ex vivo perfused lung imaging, endothelial filtration coefficient (K f) measurement, pulmonary vascular resistance measurement, Evan’s blue dye uptake, and histopathology. A PBPK model-derived measure of lung vascular permeability-surface area product increased from 2.60 ± 0.40 [CL: 2.42–2.78] mL/min in the non-irradiated group to 6.94 ± 8.25 [CL: 3.56–10.31] mL/min in 13 Gy gro...
Journal of visualized experiments : JoVE, 2013
This method is based on the intravenous injection of Evans Blue in mice as the test animal model. Evans blue is a dye that binds albumin. Under physiologic conditions the endothelium is impermeable to albumin, so Evans blue bound albumin remains restricted within blood vessels. In pathologic conditions that promote increased vascular permeability endothelial cells partially lose their close contacts and the endothelium becomes permeable to small proteins such as albumin. This condition allows for extravasation of Evans Blue in tissues. A healthy endothelium prevents extravasation of the dye in the neighboring vascularized tissues. Organs with increased permeability will show significantly increased blue coloration compared to organs with intact endothelium. The level of vascular permeability can be assessed by simple visualization or by quantitative measurement of the dye incorporated per milligram of tissue of control versus experimental animal/tissue. Two powerful aspects of this ...
Clinical Science, 2008
Capillary leak accompanying systemic inflammatory response conditions is a significant clinical problem. In the present study, we describe and verify a method for studying capillary leak that is based on the injection of proteins that differ significantly in size and have spectrally distinguishable fluorophores. Control (n=11) and post-CLP (caecal ligation and puncture; n=14) Sprague–Dawley rats were injected with tracer amounts of albumin and PEG–Alb [albumin covalently linked to methoxy-poly(ethylene glycol)] labelled with fluorescein and Texas Red. Blood samples were withdrawn between 5 min and 144 h, and the fluorescence of the labelled proteins was determined. The relative retention of the PEG–Alb and albumin was assessed via measurement of the TER (transcapillary escape rate; in %/h) and the t50% estimate, defined as the time when the actual concentration reached 50% of its baseline. The concentration–time trends for both albumin and PEG–Alb tracers exhibited two-compartmental...
Thrombosis and Haemostasis, 2012
Vascular hyperpermeability contributes to morbidity in inflammation. Current methodologies for in vivo assessment of permeability based on extravasation of Evans Blue (EB)-bound albumin are cumbersome and often lack sensitivity. We developed a novel infrared fluorescence (IRF) methodology for measurement of EB-albumin extravasation to quantify vascular permeability in murine models. Vascular permeability induced by endotoxaemia was examined for all solid organs, brain, skin and peritoneum by IRF and the traditional absorbance-based measurement of EB in tissue extracts. Organ IRF increased linearly with increasing concentrations of intravenous EB (2.5-25 mg/kg). Tissue IRF was more sensitive for EB accumulation compared to the absorbance-based method. Accordingly, differences in vascular permeability and organ EB accumulation between lipopolysaccharide-treated and saline-treated mice were often significant when analysed by IRF-based detection but not by absorbance-based detection. EB was detected in all 353 organs analysed with IRF but only in 67% (239/353) of organs analysed by absorbance-based methodology, demonstrating improved sensitivity of EB detection in organs with IRF. In contrast, EB in plasma after EB administration was readily measured by both methods with high correlation between the two methods (n=116, r2=0.86). Quantitation of organ-specific EB-IRF differences due to endotoxin was optimal when IRF was compared between mice matched for weight, gender, and age, and with appropriate corrections for organ weight and EB plasma concentrations. Notably, EB-IRF methodology leaves organs intact for subsequent histopathology. In summary, EB-IRF is a novel, highly sensitive, rapid, and convenient method for the relative quantification of EB in intact organs of treatment versus control mice.
Journal of Biochemical and Biophysical Methods, 2007
Vascular permeability is a pathologic process in many disease states ranging from metastatic progression of malignancies to ischemiareperfusion injury. In order to more precisely study tissue, and more specifically cell layer permeability, our goal was to create a fluorescencebased assay which could quantify permeability without radioactivity or electrical impedance measurements. Human aortic endothelial cells were grown in monolayer culture on Costar®-Transwell® clear polyester membrane 6-well cell culture inserts. After monolayer integrity was confirmed, vascular endothelial growth factor (VEGF 165 ) at varying concentrations with a fixed concentration of yellow-green fluorescent 0.04 μm carboxylate-modified FluoSpheres® microspheres were placed in the luminal chamber and incubated for 24 h. When stimulated with VEGF 165 at 20, 40, 80, and 100 ng/ml, this assay system was able to detect increases in trans-layer flux of 8.2 ± 2.4%, 16.0 ± 3.7%, 41.5 ± 4.9%, and 58.6 ± 10.1% for each concentration, respectively. This represents the first fluorescence-based permeability assay with the sensitivity to detect changes in the permeability of a cell layer to fluid flux independent of protein flux; as well as being simpler and safer than previous radioactiveand impedance-based permeability assays. With the application of this in vitro assay to a variety of pathologic conditions, both the dynamics and physiology relating to cellular permeability can be more fully investigated.
Journal of General Physiology, 1996
A B STRA CT A surface fluorescence method was developed to measure transalveolar transport of water, protons, and solutes in intact perfused lungs. Lungs from c57 mice were removed and perfused via the pulmonary artery (~2 mi/min). The airspace was filled via the trachea with physiological saline containing a membrane-impermeant fluorescent indicator (FITC-dextran or aminonapthalene trisulfonic acid, ANTS). Because fluorescence is detected only near the lung surface due to light absorption by lung tissue, the surface fluorescence signal is directly proportional to indicator concentration. Confocal microscopy confirmed that the fluorescence signal arises from fluorophores in alveolijust beneath the pleural surface. Osmotic water permeability (t f) was measured from the time course of intraalveolar FITC-dextran fluorescence in response to changes in perfusate osmolality. Transalveolar Pf was 0.017 _+ 0.001 cm/s at 23~ independent of the solute used to induce osmosis (sucrose, NaC1, urea), independent of osmotic gradient size and direction, weakly temperature dependent (Arrhenius activation energy 5.3 kcal/mol) and inhibited by HgC12. Pf was not affected by cAMP activation but was decreased by 43% in lung exposed to hyperoxia for 5 d. Diffusional water permeability (Pd) and Pf were measured in the same lung from intraalveolar ANTS fluorescence, which increased by 1.8-fold upon addition of 50% I)20 tO the perfusate. Pd was 1.3 • 10 -~' cm/s at 23~ Transalveolar proton transport was measured from FITC-dextran fluorescence upon switching perfusate pH between 7.4 and 5.6; alveolar pH half-equilibrated in 1.9 and 1.0 rain without and with HCO3-, respectively. These results indicate high transalveolar water permeability in mouse lung, implicating the involvement of molecular water channels, and establish a quantitative surface fluorescence method to measure water and solute permeabilities in intact lung. Key words: alveolus * osmosis * fluorescent indicator 9 aquaporin 9 water channel
Journal of Visualized Experiments, 2013
This method is based on the intravenous injection of Evans Blue in mice. Evans blue is a dye that binds albumin. Under physiologic conditions the endothelium is impermeable to albumin, so Evans blue bound albumin remains restricted within blood vessels. In pathologic conditions that promote increased vascular, permeability endothelial cells partially lose the close contact and the endothelium becomes permeable to small proteins such as albumin. This condition allows for extravasation of Evans Blue in tissues.
Journal of Controlled Release, 2002
The overall pulmonary disposition of various fluorescent probes was viewed by confocal imaging following intratracheal delivery in the rat in vivo. The green fluorescent dyes, coumarin-6, a 350 Da lipophilic molecule; calcein, a 623 Da hydrophilic molecule; or FITC-albumin, a 65 000 Da hydrophilic molecule; were insufflated as a dry powder or instilled as a solution in the lungs of rat in vivo. Immediately, 2 or 24 h following delivery, the lungs were colored with sulforhodamine and fixed by vascular perfusion. The lungs were then removed, grossly sliced and examined by confocal laser scanning fluorescence microscopy. Coumarin-6 diffused within minutes across the trachea, airways and alveolar tissue but was also retained for hours in type II alveolar epithelial cells. The diffusion of calcein across the tissue was fast as well, with no particular affinity for specific cells. FITC-albumin slowly permeated the tissue. It remained in the airspaces for hours and was intensively captured by alveolar macrophages. Compared to the powder, the solution bypassed dissolution and therefore shortened the lag time for diffusion and cellular capture. The technique allowed to obtain an overview of the fate of fluorescent probes locally in each region of the lungs and highlighted the strong dependence of the localization behavior on physico-chemical properties of molecules as well as a capture by particular cells of the pulmonary tissue.
Laboratory Investigation, 2010
We present a new lung imaging technique based on endoscopic confocal fluorescence microscopy (ECFM), which is a new method that is able to provide cellular and structural assessment of living tissue using a small confocal probe in direct contact with the visceral pleura. To observe distal airspace structure and cellular condition in normal and injured lungs (hyperoxic and bleomycin challenged), we used fluorescent-specific marker contrast and ECFM. Alveolar space ECFM with spectral analyses were performed at 488-nm excitation using FITC-labeled markers or naturally fluorescent dyes. The normal lung was compared with the sick lung, where our in vivo imaging experiments correlated well with results obtained with corresponding ex vivo conventional assays. Four main elements pertaining to the acute lung injury/ acute respiratory distress syndrome (ALI/ARDS) pathophysiology and established early key events were specifically studied: alveolar epithelial membrane phenotype, lung cell apoptosis, neutrophil recruitment, and edema. ECFM allowed visualization of (i) fine-tuned ultrastructural lectin (RCA-1) and sialoglycoprotein (RTI40) epithelial cell membrane expression, (ii) YO-PRO-1-related DNA linking of lung cell apoptosis, (iii) PKH2 green fluorescent cell linker-labeled neutrophil tracking in lung microcirculatory network and airspaces, (iv) FITC-dextran plasma contrast and extravasation with edema formation. ECFM provides reliable results to corresponding ex vivo fluorescent methods. ECFM, using the minimally invasive Five-1s optical instrument and specific fluorescent markers, is able to provide real-time potentially useful imaging of live unfixed normal and injured lung tissue with promising developments for improving bedside diagnostic and decision-making therapeutic strategy in patients with ALI.
Journal of Surgical Research, 1979

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
References (284)
- Singer, G., et al. Microcirculation 13(2):89-97, 2006. References
- Neri M et al. Mediators Inflamm. 2016:3423450, 2016
- Sato R et al. J Intensive Care. 3:48, 2015
- De Acetis M et al. Circ Res. 96(10):1087-94, 2005
- Catalucci D et al. J Cell Biol. 184(6):923-33, 2009. References
- Wilhagen et al, Blood 123:1098-1101, 2014
- Xu et al, Nat Med 15:1318-1321, 2009 [3]Thromb Res 136:542-7, 2015
- Beloborodova NV. Sepsis. Chapter 1. 2017. DOI: 10.5772/68046
- McDonald D et al. mSphere 1(4): e00199-16, 2016
- Jenner AM et al. Free Radic Biol Med 38:763-772, 2005 Reference
- Brodska H et al. Clin Exp Med 13(3):165-70, 2013. References
- Hwang SY et al. Am J Emerg Med 35(2):234-239, 2017.
- Salciccioli JD et al. Crit Care 19:13, 2015. References
- Boeken U, et al. Cardiovasc Surg 8(7):550-4, 2000.
- Prat C, et al. J Cardiac Surg 23(6):627-32, 2008 References
- Fukushima H et al. Shock 39(5):409-14, 2013.
- Azfar MF et al. Clin Invest Med 40(2):E49-E58, 2017.
- Gossett DR et al. PNAS 20:7630-5, 2012
- Crawford K et al. AJRCCM, under review, 2017
- Yunxiang Li. Int J Clin Exp Pathol 9:11904-11910, 2016 Reference 1. WHO Guidelines on Hand Hygiene in Health Care. 2009
- Ulger F et al. Ann Clin Microbiol Antimicrob 8:7, 2009
- Russotto V et al. J Intensive Care 3:54, 2015 References
- Burgmann H et al. Intensive Care Med 36:1597-1601, 2010.
- Boncagni F et al. Minerva Anestesiol 81:765-775.3, 2015. References
- Craig WA et al. Antimicrob Agents Chemotherap 36(12):2577-83, 1992
- Roberts JA et al. Am J Respir Crit Care Med 194(6):681-91, 2016 Reference
- Mongardon N et al Crit Care Med 39:1359-64, 2011.
- Davies KJ et al Resuscitation 84:616-9, 2013 References
- Oberoi JK JIMSA 23 No. 1, January -March 2010
- P070 Incidence of Trichosporon spp. urinary tract infections in ICU E Belesiotou, C Routsi, C Vrettou, E Magira, E Douka, P Kaltsas, M Nepka, E Perivolioti, E Kraniotaki, S Zakinthinos Evaggelismos General HOSPITAL, Athens, Greece Critical Care 2018, 22(Suppl 1):P070 References
- Coetzee J et al. 106:449-450, 2016
- Morrissey I et al. Pharmaceuticals. 6(11):1335-1346, 2013
- McGuinness WA et al. Yale Bio Med 90(2):269-281, 2017
- Knapp S, et al. Am J Respir Crit Care Med. 1;173(1):122-9, 2006
- Merabishvili M, et al. PLoS One. 11;9(8):e104853, 2014 References
- Giamarellos-Bourboulis EJ et al. J Antimicrob Chemother 69: 1111-8, 2014
- Giamarellos-Bourboulis EJ et al. Clin Infect Dis 46: 1157-64, 2008
- Bassetti M et al. Intensive Care Med. 44:73-75, 2017
- P089 Experience of having no set criteria for an outreach (rapid response) team in a new hospital H Tan, J Teh, F Lee, G Soo, N Horsahamay, S Tan, Y Lee, F Khan Ng Teng Fong General Hospital, Singapore, Singapore Critical Care 2018, 22(Suppl 1):P089
- Rhodes A et al. Intensive Care Med 43:304-377,2017
- Michael D et al. JAMA 317:847-848,2017 References
- Rhodes et al. Crit Care Med 45:486-552, 2017
- Khanna AK et al. SCCM 2018 (Abstract #177) References
- Vincent JL et al. Can Respir J 2017: 2834956, 2017
- Hafner S et al. 5:42. 2015
- Johnson AEW et al. Scientific Data 3:160035, 2016
- Shankar-Hari M et al. JAMA 315(8):775-787, 2016. P109 Apoptotic cells induce cytokine homeostasis following LPS treatment D Mevorach Hadassah-Hebrew University, Jerusalem, Israel Critical Care 2018, 22(Suppl 1):P109
- Nakamura Y et al. Crit Care 21(1):134, 2017
- Garg SK et al. Critical Care Research and Practice, 3635609:1-10, 2017 Reference
- Khanna et al. N Engl J Med; 377(5):419-430, 2017 References
- Cholley B et al. JAMA 318:548-556, 2017
- Landoni G et al. N Engl J Med 376(21):2021-2031, 2017
- Mehta RH et al. N Engl J Med 376(21):2032-2042, 2017
- Harrison RH et al. J Cardiothorac Vasc Anesth 27:1224-1232, 2013
- Sanfilippo F et al. Crit Care 21(1):252, 2017
- Chen QH et al. Crit Care 21(1):253, 2017 References
- Hajjar LA et al. Anesthesiology 126:85-93, 2017
- Russell JA et al. N Engl J Med 358:877-87, 2008 P130 The effect of norepinephrine on venous return during VA-ECMO A Hana 1 , PW Moller 2 , PP Heinisch 1 , J Takala 1 , D Berger 1 Reference
- Knight M et al. MBRRACE-UK Saving Lives, Improving Mothers' Care 2016. P133 Real-time guidance or prelocation using ultrasound for pediatric central venous catheterization; a systematic review and network meta-analysis K Hosokawa, N Shime, M Kyo, Y Iwasaki, Y Kida Hiroshima University Hospital, Hiroshima, Japan Critical Care 2018, 22(Suppl 1):P133
- References Hasin T et al. J Am Coll Cardiol 61(2):153-63, 2013
- Tsiouris A et al. J Heart Lung Transplant 33(10):1041-7, 2014
- Forest SJ et al. Ann Thorac Surg 95(4):1276-81, 2013 References
- Pedrosa J et al. Curr Pharm Des 22:105-21, 2016
- Critchley L et al. J Clin Monit Comput 15:85-91, 1999
- Journal of Hepatology 2015 vol. 63 j 634 ̈C642 References
- Borg R et al. Intensive Care Soc 18:184-192, 2017.
- Borthwick M et al. Int Pharm Pract (in press), 2017.
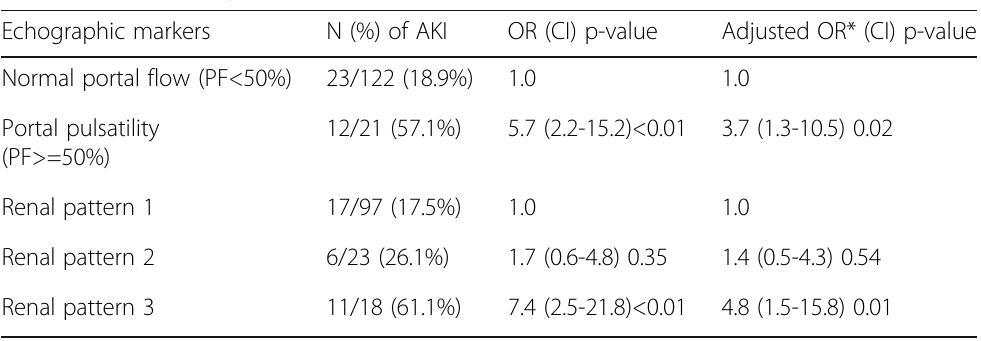
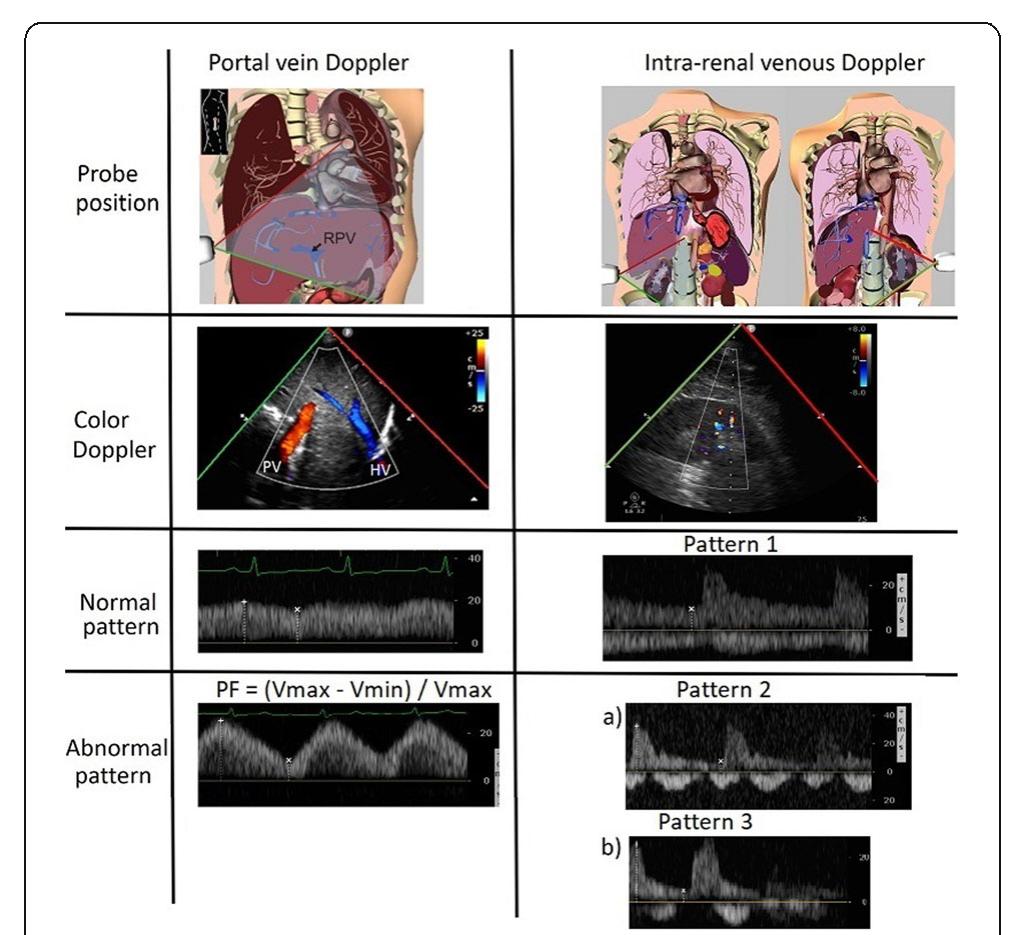
- Kidney Disease: Improving Global Outcomes Acute Kidney Injury Working Group. Kidney Int Suppl 2:1-138, 2012. Reference
- Schwarzer P. et al, Crit Care. 2015 Sep 8;19:321 P156 Withdrawn P157 Association of pain in the critically ill patient with acute kidney injury risk in the intensive care unit (ICU)
- JM Vieira Junior, LB Herranz, LC Pontes de Azevedo, I Castro Hospital Sírio Libanês, Sao Paulo, Brazil Critical Care 2018, 22(Suppl 1):P157
- Coca SG et al. Am J Kidney Dis 53(6):961-73, 2009
- P159 Loop diuretics to treat acute kidney injury in critically ill patients: a systematic review and meta-analysis K Rosas 1 , D Gutierrez 2 References
- Hoste EAJ et al. Intensive Care Med 41(8):1411-1423, 2015
- Hoste AJ et al. Crit Care 10(3):R73, 2006
- Kaddourah A et al. N Engl J Med 376:11-20, 2017
- ClinicalTrials.gov Identifier: NCT01706497 References
- Hoste EA, et al. Intensive Care Med 41(8):1411-23, 2015
- Lannemyr L et al. Anesthesiology 126(2):205-213, 2017 P163 Utility of daily creatine kinase measurement within adult intensive care P Henderson 1 , J Adams 2 , A Blunsum 1 , M Casey 3 , N Killeen 1 , S Linnen 1 , J McKechnie 3 , A Puxty 1
- Glasgow Royal Infirmary, Glasgow, UK; 2 Royal Alexandra Hospital, Paisley, UK;
- Hobson C et al. Ann Surg 261:1207-14, 2015
- Maciel AT et al. Renal Failure 38(10):1607-15, 2016
- Acute Kidney Injury Work Group: Kidney Int; 2:1-138, 2012 P168 Urinary liver-type fatty acid-binding protein is the novel biomarker for diagnosis of acute kidney injury secondary to sepsis T Komuro, T Ota Shonan Kamakura General Hospital, Kamakura, Kanagawa, Japan Critical Care 2018, 22(Suppl 1):P168
- Susantitaphong P et al. Am J Kidney Dis 61(3):430-9, 2013
- Singer M et al. JAMA 23;315(8):801-10, 2016
- P169 Vitamin D metabolite concentrations in critically ill patients with acute kidney injury L Cameron, U Blanco Alonso, A Bociek, A Kelly, G Hampson, M Ostermann Guy's and St Thomas' NHS Foundation Trust, London, UK Critical Care 2018, 22(Suppl 1):P169 Reference
- Werle RW et al. CoDAS 28: 646-652, 2016. References
- Choi HJ et al. Emerg Med J 27(5):380-2, 2010
- Mosier JM et al. J Emerg Med 42(6):629-34, 2012. P176 10-year cohort of prehospital intubations and rescue airway techniques by helicopter emergency medical service physicians: a retrospective database study P De Jong, C Slagt, N Hoogerwerf Radboudumc, Nijmegen, Netherlands Critical Care 2018, 22(Suppl 1):P176 References
- Slagt C et al. Air Med J 23:36-7, 2004
- Peters J et al. Eur J Emerg Med 22:391-4, 2015 References Templier F et al. Eur J Emerg Med 10(2):87-93, 2003
- Mao C et al. Crit Care Med 27(12):2806-2811, 1999 P196 Diaphragm ultrasound to predict weaning outcomes in mechanically ventilated patients S Abdallah, A Ben Souissi, W Yaakoubi, S Kamoun, I Ben Naoui, MS Mebazaa Mongi Slim university Hospital, Tunis, Tunisia Critical Care 2018, 22(Suppl 1):P196
- References Mayo P et al. Intensive Care Med 42:7, 2016 References [1] ARDSNet 2000, [2] Bein 2013 [3] Terragni 2009 [4] Forster 2013 References 1. Crit Care Med 2015;43(12):2570-81
- Anaesth Crit Care Pain Med 34;(2015):135-140 References
- Giannoni A et al. J Am Coll Cardiol 53:1975-80, 2009
- Trembach N et al. Respir Physiol Neurobiol 235:79-82, 2017
- Chua TP et al. Eur Clin Invest 25:887-92, 1995 P209A Outcomes of patients admitted to the intensive care unit with acute exacerbation of pulmonary fibrosis.
- S Sahota, J Paddle, C Powell Royal Cornwall Hospital, Truro, UK Critical Care 2018, 22(Suppl 1):P209A
- Wells et al. Thorax 63. Suppl 5 v1-v58, 2008
- NICE Clinical Guidelines: Idiopathic pulmonary fibrosis in adults: diagnosis and management (June 2013) www.nice.org.uk, accessed 15 Nov 2017 P210 Acute respiratory failure (ARF): differences in diaphragmatic ultrasonography in medical and surgical patients G Zani, F Di Antonio, M Pichetti, V Cricca, A Garelli, C Gecele, M Campi, F Menchise, M Valbonetti, S Mescolini, E Graziani, M Diamanti, C Nencini, FD Baccarini, M Fusari Santa Maria delle Croci Hospital, Ravenna, Italy Critical Care 2018, 22(Suppl 1):P210 References
- Zambon et al Annual Update in Inten Care and Emerg Med 427-438, 2013
- Umbrello M et al. Respir Care 2016 References
- Lerolle N et al. Chest 2009
- Houston JG et al. Clin Radiol 1992 P215 Esophageal pressure and diaphragm A Menis, D Makris, V Tsolaki, E Zakynthinos University Hospital of Larisa, Larissa, Greece Critical Care 2018, 22(Suppl 1):P215 Reference
- Supinski G et al. Chest, 2017 References P218 Electrical impedance tomography guided ventilation R Knafelj, M Noc, V Gorjup University Medical Center Ljubljana, Ljubljana, Slovenia Critical Care 2018, 22(Suppl 1):P218 References
- Amato MB et al. N Engl J Med. 372(8):747-55, 2015
- Younes M et al. Am J Respir Crit Care Med. 164(1):50-60, 2001 P222 Comparison of respiratory volume monitoring vs. capnography during intravenous propofol-based anesthesia JE Freeman 1 , S Pentakota 2 , E Blaney 2 , B Kodali 2
- 1 Respiratory Motion, inc., Waltham, MA, USA; 2 Brigham and Women's Hospital, Boston, MA, USA Critical Care 2018, 22(Suppl 1):P222
- Xu XP et al. Stem Cell Res Ther 8:164, 2017.
- P227 Hepatocyte growth factor protects against lipopolysaccharide-induced endothelial barrier dysfunction with Akt/mTOR/STAT-3 pathway S Meng, F Guo, X Zhang, W Chang, H Qiu, Y Yang Department of Critical Care Medicine, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, China Critical Care 2018, 22(Suppl 1):P227 References
- Repessé et al. Chest 147.1: 259-265, 2015.
- Fumagalli et al. Crit Care Med 45.8: 1374-1381, 2017.
- P243 Heparin-binding protein in ventilator induced lung injury J Tyden 1 , N Larsson 1 , H Herwald 2 , M Hultin 1 , J Wallden 1 , J Johansson 1 1 Umea university, Umea, Sweden; 2 Lund university, Lund, Sweden Critical Care 2018, 22(Suppl 1):P243
- Slutsky AS et al. N Engl J Med 369(22):2126-2136, 2013
- Johansson J et al. Acta Anaesthesiol Scand 57(5):580-586, 2013
- Bentzer P et al. Intensive Care Med Exp 4(1):33, 2016
- Table 1 (abstract P242). Ventilatory setting, oxygenation, mechanics and echocardiography measurements Baseline (n=17) Incremental (n=17) Decremental (n=17)
- PaO2/FiO2= arterial partial pressure of oxygen to inspired fraction of oxygen ratio. PEEP=Positive End Expiratory Pressure, TAPSE (Tricuspid annular plane systolic excursion) and S' (Velocity of the tricuspid annular systolic motion). All the results are shown as Average ± Standard Deviation. * significant compared to Baseline p<0.05; ^significant compared to Incremental p<0.05. References Meduri GU et al. Chest 131(4):954-63, 2007
- Gu WJ et al. Chest. 2016 ;149:166-79.
- Tang L et al. PLoS One. 2014;9:e111527. P267 Comparison of real time ultrasound guidance versus palpation technique in radial artery catheterization in critically ill patients presenting with hypotension: a randomized controlled trial MS Khan, S Myatra, V Bhagat, S Siddiqui, A Narkhede, N Prabhu, AP Kulkarni, J Divatia Tata Memorial Hospital, Mumbai, India Critical Care 2018, 22(Suppl 1):P267
- Slagt C et al. Crit Care 14:208, 2010
- Cecconi M et al. Crit Care 13:201, 2009
- Critchley LA et al. J Cardiothorac Vasc Anesth 25:536-46, 2011 Reference
- Vincent JL et al. Crit Care 19:251, 2015
- P284 A reappraisal of the effects of fluid administration on left ventricular loading conditions in critically ill patients M Jozwiak 1 , S Millasseau 2 , C Richard 1 , X Monnet 1 , P Mercado 1 , F Dépret 1 , JE Alphonsine 1 , JL Teboul 1 , D Chemla 3
- 1 Hôpitaux universitaires Paris-Sud, Hôpital de Bicêtre, APHP, service de réanimation médicale; Inserm UMR S_999, Univ Paris-Sud, Le Kremlin- Bicêtre, France, 2 Pulse Wave Consuting, Saint Leu La Foret, France, 3 Hôpitaux universitaires Paris-Sud, Hôpital de Bicêtre, APHP, service de physiologie; Inserm UMR S_999, Univ Paris-Sud, Le Kremlin-Bicêtre, France Critical Care 2018, 22(Suppl 1):P284
- Blank R, Napolitano L. Crit Care Clin 2011;27:439-58.
- Ashbaugh DG, Bigelow DB, Petty TL, et al. Lancet 1967;2:319-23.
- Murray J, Matthay M, Luce J, et al. Am Rev Respir Dis 1988;138:720-3.
- P292 Association between fluid overload and SOFA score kinetic from admission to day 5 during septic shock: results of EPIGOAL study X Chapalain 1 , V Vermeersch 1 , P Egreteau 2 , J Oilleau 1 , G Prat 1 , O Huet 1
- Wilson RF et al. J Trauma Acute Care Surg 74:45-50, 2013 P301 Effects of crystalloid vs. colloid volume replacement on microcirculatory perfusion in free flap surgery I László 1 , Á Janovszky 1 , N Öveges 1 , Z Lóderer 2 , P József 1 , A Szabó 1 , V Vargán 1 , A Lovas 1 , T Tánczos 1 , A Mikor 1 , D Trásy 1 , Z Molnár 1 1 University of Szeged, Szeged, Hungary, 2 Markusovszky Hospital, Szombathely, Hungary Critical Care 2018, 22(Suppl 1):P301
- Molnar Z et al. Curr Opin Anaesthesiol 171-177, 2017 P302 Deciphering the immunomodulatory consequences of crystalloids F Brillant-Marquis, JA Sauvé, P Laplante, P Thebault, M Chassé, FM Carrier, R Lapointe, JF Cailhier CRCHUM/CHUM/Université de Montréal, Montreal, Canada Critical Care 2018, 22(Suppl 1):P302 References
- Annane D et al. JAMA 310:1809-17, 2013
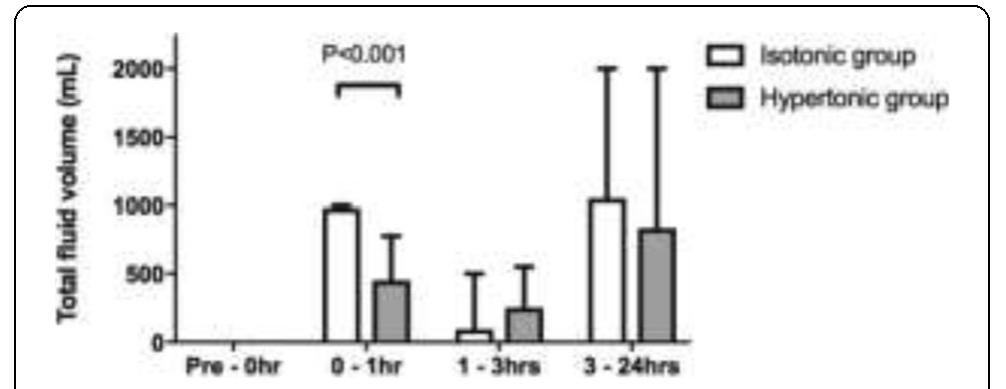
- Ip WK et al. Nat Commun 6:6931, 2015 P303 Hypertonic versus isotonic saline for initial fluid bolus in emergency department patients with suspected infection: a pilot RCT L Smart, E Bosio, S Macdonald, D Fatovich, C Neil, G Arendts Harry Perkins Institute of Medical Research, Perth, Australia Critical Care 2018, 22(Suppl 1):P303
- Staempfli HR et al. J Appl Physiol 95:620-630, 2003
- Van Slyke DD J Biol Chem 52:525-570, 1922
- Langer T et al. J Crit Care 30(1):2-6, 2015 P305 Impact of electrolyte imbalance on prognosis in patients with aneurysmal subarachnoid haemorrhage: a retrospective study CWY Tam, HP Shum Pamela Youde Nethersole Eastern Hospital, Hong Kong, China Critical Care 2018, 22(Suppl 1):P305 Reference
- Dean P et al. Crit Care 17:443, 2013 P307 Effect of hypernatraemia recorded within the first 24 hours on length of stay A McManus, T Samuels, A Myers, P Morgan East Surrey Hospital, Redhill, UK Critical Care 2018, 22(Suppl 1):P307
- Felder S et al. J Surg Educ. 71(5):768-773, 2014. P309 Stress ulcer prophylaxis in critically ill patients; when to stop? Y Kato, H Uchino, T Kaihara, T Fukuoka Kurashiki Central Hospital, Kurashiki Okayama, Japan Critical Care 2018, 22(Suppl 1):P309
- Saunders DI et al. Br J Anaesth 109(3):368-375, 2012 P311 Injury caused by colorectal surgery: the role of proteins S100A J Maca, P Ihnat, M Peteja, O Jor, P Reimer, P Sevcik University Hospital od Ostrava, Ostrava, Czech Republic Critical Care 2018, 22(Suppl 1):P311 References
- Compher C et al. Crit Care Med 45:156-163, 2017
- Yeh DD et al. JPEN 40:37-44, 2015
- Nicolo M et al. JPEN 40:756-762, 2015
- Ferrie S et al. JPEN 40:795-895, 2015
- Heyland DK et al. JPEN 27:74-83, 2003
- Cahill NE et al. Critical Care Medicine 38:395-401, 2010. References
- Yoshimoto Y et al. Stroke 32:1989, 2001
- Calder PC et al. Clin Nutr 30:1, 2017 References
- Casaer MP et al. N Eng J Med 365(6): 506-17, 2011
- Vanderheyden S et al. Crit Care 16(3): R96, 2012 P330 Vitamin C supplementation in the critically ill: systematic review and meta-analysis P Laferriere-Langlois 1 , F Lamontagne 1 , W Manzanares 2
- CHUS, Sherbrooke, Canada, 2 Hospital de Clinicas, Montevideo, Uruguay Critical Care 2018, 22(Suppl 1):P330
- McClave S et al. Enteral Nutr 40:159-211, 2016 P332 Energy requirements of the critically ill patient during the first week R Marinho 1 , R Sousa 2 , R Sousa 2 , M Lopes 1 , M Santos 3 , A Marinho 1 Reference
- McClave SA et al. JPEN J Parenter Enteral Nutr. 40(2):159-211, 2016 P334 Comparison of nutric score, nutritional risk screening (NRS) 2002 and subjective global assessment (SGA) in the ICU: a cohort study S Saseedharan 1 , EJ Pathrose 1 , DR Karnad 2 , AT Patil 1
- S L Raheja Hospital, Mumbai, India, 2 Jupiter Hospital, Thane, India Critical Care 2018, 22(Suppl 1):P334 References
- Correia MI, Campos AC, Study EC. Nutrition 19(10):823-5, 2003
- Waitzberg DL, Caiaffa WT, Correia MI. Nutrition17(7):573-80, 2001 References
- Zusman O, Singer P. Crit Care 21(1):128, 2017
- P337 Both the immediate and delayed inflammatory responses in all major organs are reduced by single dose 17β-estradiol following severe burn injury JG Wigginton, PE Pepe, KR AbdelFattah, JW Gatson, AH Idris, V Warren, JP Minei, DL Maass UT Southwestern Medical Center, Dallas, Texas, USA Critical Care 2018, 22(Suppl 1):P337 References
- Walker JJ et al. Proc Biol Sci. 277:1627-33, 2010
- Conway-Campbell BL et al. J Neuroendocrinol. 22:1093-100, 2010 References
- Savage M., et al., Diabetic Med 28(5):508-515, 2011
- P347 Maximal glycemic gap is the best glycemic variability index to ICU mortality in medical critically ill patients T Issarawattna, R Bhurayanontachai Prince of Songkla University, Songkla, Thailand Critical Care 2018, 22(Suppl 1):P347 References
- Jemal A et al., J Natl Cancer Inst, 109, 2017
- Johnson AEW et al., Sci Data, 160035, 2016
- Singer et al. JAMA 315(8):801-810, 2016
- P353 Enoxaparin pharmacokinetics in with augmented renal clearance, preliminary results of a single center study A Ramos 1 , A Dogliotti 2 , N Pires 1 , C Lovesio 1 , D Latasa 1 , M Perezlindo 1 , F Acharta 1
- Sanatorio Parque, Rosario, Argentina, 2 Grupo Oroño, Rosario, Argentina Critical Care 2018, 22(Suppl 1):P353 References
- Stover EP et al. Anesthesiology 88:327-33, 1998
- Kytola L et al. Acta Anaesthesiol Scand 42:178-83, 1998 P360 Predictors of red blood cell transfusion in cardiac surgery patients D Ringaitiene, L Puodziukaite, V Vicka, D Gineityte, J Sipylaite Department of Anesthesiology and Intensive Care, Institute of Clinical Medicine, Faculty of Medicine, Vilnius University, Vilnius, Lithuania Critical Care 2018, 22(Suppl 1):P360 References
- Rocha LL et al. Trials 18:85, 2017 References
- Innerhofer P et al. Lancet Haematol 4(6):e258-e271, 2017 References
- Tonglet ML et al. Crit Care 18(6):648, 2014 References
- Pape HC et al. Damage control management in the polytrauma patient 2010, P.13-14.
- P370 Tranexamic acid in pediatric trauma JM Thomson, HM Drone, JL Jantzer, AK Tsai, JT Jancik Hennepin County Medical Center, Minneapolis, Minnesota, USA Critical Care 2018, 22(Suppl 1):P370
- CRASH-2 trial collaborators. Lancet 376:23-32, 2010. References
- Geerts et al. 1994
- Falck-Ytter et al. 2012
- Décousus et al. 1998
- Falck-Ytter et al. 2012
- Lee et al. 2015 References
- Williamson DR et al. Syst Rev 5(1):193, 2016 P377 Impact of decompressive craniectomy on neurological functional outcome in critically ill adult patients with severe traumatic brain injury: a systematic review and meta-analysis P Bonaventure, JA Jamous, F Lauzier, R Zarychanski, C Francoeur, A Turgeon CHU de Québec -Université Laval, Québec, Canada Critical Care 2018, 22(Suppl 1):P377
- Maas AIR, Menon DK, Adelson PD, et al. Lancet Neurol. November 2017. doi:10.1016/S1474-4422(17)30371-X.
- Carney N, Totten AM. Guidelines for the Management of Severe Traumatic Brain Injury. 4th ed. Brain Trauma Foundation
- Citerio G et al. Acta Neurochir (Wien). 142(7):769-776, 2000 References
- Kochanek PM et al. Pediatric Crit Care Med 13:S1-S82, 2012
- Sutherland SM et al. Clin J Am Soc Nephrol 10:554-561, 2015 References
- Gomez PA et al. J Neurosurg 121:1314-1322, 2014
- Johansen JS. Dan Med Bull 53:172-209, 2006 References
- Albertario CL et al. Sleep 18(10):836-43,1995
- Schӧnenberger S et al. Neurocrit Care 25:94-104, 2016 P391 Venous thromboembolism prophylaxis among neurocritical care patients: what is current KM Sauro 1 , DJ Niven 1 , A Soo 1 , P Couillard 1 , A Kramer 1 , J Kromm 1 , D Zygun 2 , SM Bagshaw 2 , HT Stelfox 1
- 1 University of Calgary, Calgary, Canada, 2 University of Alberta, Edmonton, Canada Critical Care 2018, 22(Suppl 1):P391 References
- Zajic P et al. Crit Care. 21(1):223, 2017 References
- Nishiyama T, et al. Can J Anaesth 47(12):1196-201, 2000 P397 Intracranial hemorrhage in HELLP syndrome patients admitted to the ICU T Yuyen, S Kongsayreepong, A Piriyapatsom Department of Anesthesiology, Siriraj Hospital, Mahidol University, Bangkok, Thailand Critical Care 2018, 22(Suppl 1):P397
- Okonkwo DO et al. Crit Care Med 45:1907-1914, 2017
- P401 Intracranial hemorrhage after thrombolysis for ischemic stroke: life threatening complication or irrational fear? G Karlis 1 , N Magkas 2 References
- Prosser J et al. Stroke 38:2295-2302, 2007 P407 Hyperkinetic disorder treatment using sevoflurane in patients with unresponsive wakefulness syndrome (UWS) and minimal conscience state (MCS)
- E Kondratieva, N Lesteva, A Kondratiev, S Kondratyev Polenov Neurosurgical Institute, Saint Petersburg, Russia Critical Care 2018, 22(Suppl 1):P407 References
- Soar et al; European Guidelines for Resuscitation; 100-147, 2015 2. Australian Resuscitation Council; Guideline 11.6; 2016 P415 Impact of phone CPR on ROSC outcome A Giugni 1 , S Gherardi 2 , L Giuntoli 1 , A Monesi 1 , A Finelli 1 , G Gordini 1 1 Maggiore Hospital, Bologna, Italy; 2 Bologna University, Bologna, Italy Critical Care 2018, 22(Suppl 1):P415
- Wnent, J. et al. Resuscitation 118: e11-e12, 2017
- Tanaka, Y. et al. Resuscitation 83: 1235-1241, 2017
- Viereck S. et al. Resuscitation 115: 141-147, 2017. References
- Kirkegaard H et al. JAMA 318:341-50, 2017 P417 Coronary angiographic findings after cardiac arrest in relation to ECG and comorbidity R Lagedal, L Elfwén, M Jonsson, E Lindgren, D Smekal, L Svensson, S James, P Nordberg, S Rubertsson Institution for surgical sciences, anaesthesia and intensive care, Uppsala, Sweden Critical Care 2018, 22(Suppl 1):P417 References
- Young P et al. Resuscitation 85(12):1686-1691, 2014
- Applegate R et al. Anesth Analg 123(3):626-633, 2016 P422 Implementation of a rescue and organ perfusion protocol after ohca: clinical experience and perspective A Franci, G Di Tommaso, G Cianchi, ML Migliaccio, C Guetti, M Ciapetti, S Di Valvasone, M Bonizzoli, A Peris Azienda Ospedaliero Universitaria Careggi, Firenze, Italy Critical Care 2018, 22(Suppl 1):P422 Reference
- Windecker S, Kolh P et al. Eur Heart J 35:2585, 2014
- Hawkes C, et al. Resuscitation 110:133-140, 2017
- P433 Outcomes of patients admitted to intensive care following cardiac arrest J McLoughlin, E Landymore, P Morgan East Surrey Hospital, Surrey, UK Critical Care 2018, 22(Suppl 1):P433 References
- Nolan JP et al. Resuscitation 85:987-992. 2014 References
- Haesen J et al. Crit Care 19(Suppl 1):434, 2015. References
- Unverir et all, Human & Experimental Toxicology 26: 757-761, 2007 P439 Predictive factors for secondary ICU admission within 48 hours after hospitalization in a medical wards from the emergency room M Cancella De Abreu 1 , S Herminger 1 , A Rousseau 2 , P Hausfater 1
- Hôpital Pitié Salpêtrière, Paris, France; 2 Hôpital Saint Antoine, Paris, France Critical Care 2018, 22(Suppl 1):P439
- Mariyaselvam et al. Anesthesiology. 127: 658-65, 2017
- https://nhsaccelerator.com/innovation/the-wiresafe/ P441 Acquired neuromuscular weakness in eldery patients with femoral bone fracture, could we decrease the incidence? D Pavelescu, I Grintescu, L Mirea Emergency Hospital Floreasca, Bucharest, Romania Critical Care 2018, 22(Suppl 1):P441 References
- Ebbeling L et al. Eur J Trauma Emerg Surg 40(1):57-65, 2014 P443 Rapid ultrasonographic assessment of undifferentiated shock in hypotensive patients E Tesfaye, T Zewude Tikur Anbessa Specialised Hospital, Addis Ababa, Ethiopia Critical Care 2018, 22(Suppl 1):P443
- Volpicelli G et al. Intensive Care Med 39:1290-8, 2013. References
- Arbon P et al. Prehosp Disast Med 16(3):109-116, 2001. P445 Adasuve enables quicker dispositions of acute psychiatric patients in the emergency department K Hesse 1 , E Kulstad 2 , K Netti 1 , D Rochford 1 , R Shah 1
- Advocate Healthcare, Oak Lawn, USA; 2 UT Southwestern, Dallas, USA Critical Care 2018, 22(Suppl 1):P445
- Dawson et al. Sl Med Rev 365-80, 2005 References
- Donaldson LJ et al. The Lancet 389:1680-1682, 2017
- Henneman EA et al. Applied Nursing Research 23:11-21, 2010
- Whitehair L et al. Nurse Education Today 34:225-232, 2014
- Mariani B et al. Clinical Simulation in Nursing 13(5):210-216, 2017 References
- Walpot H et al.: Anaesthesist, 1989
- Jack T et al Intensive Care Med, 2012
- European Directorate for the Quality of Medicines & HealthCare of the Council of Europe. Guide to the quality and safety of organs for transplantation. 6th edition. 39-46, 2016.
- Richard V et all. Transplant coordination manual. 3rd edition. 37-43, 2014. P459 Current status and problems of organ transplantation before and after the enactment of the revised organ transplant law in Japan S Kamada, T Ikeda, S Ono, S Suda, T Nagura Tokyo Medical University, Hachioji Medical Center, Tokyo, Japan Critical Care 2018, 22(Suppl 1):P459
- Drew C. et al NEJM 368:2075-2083, 2013
- P461 A hug bid a day keeps the patient ok! E Sousa, T Leonor, R Pinho Centro Hospitalar de Entre Douro e Vouga, Santa Maria da Feira, Portugal Critical Care 2018, 22(Suppl 1):P461 References
- Williams, J. et al. Anaesthesia 70, 32-40 (2015).
- Mathews, K. S. et al. Ann. Am. Thorac. Soc. 12, 886-894 (2015).
- Kroese, D. P. et al. Wiley Interdiscip. Rev. Comput. Stat. 6, 386-392 (2014).
- Lone, N. I. et al. Crit. Care 15, R102 (2011).
- Fig. 1 (abstract P463). Monte Carlo simulations of bed occupancy under 1) normal, 2) increased referral, 3) increased referral and increased capacity conditions References
- Preoperative Medicine the Pathway to Better Surgical Care. London: Royal College of Anaesthetists; 2015.
- P465 Morale: introducing the anaesthetic trainee confession session J Cuddihy, G Sivasubramaniam, D Mahtani, V Ponnaiah, V Ponnaiah, E Aziz Guys and St Thomas' Hospital, London, UK Critical Care 2018, 22(Suppl 1):P465
- Varon J et al. Curr Opin Crit Care 8(6):616-24, 2002 References
- Kerr et al, Occup Med 59:574-579, 2009 P476 Introduction of a structured debrief on intensive care: a quality improvement initiative to optimise learning and improve practice L Zucco, H Damirji University Hospital Lewisham, London, UK Critical Care 2018, 22(Suppl 1):P476 References
- Ahmed M, et al. Ann Surg 258(6):958-63, 2013
- Kalfon P et al, Intensive Care Med 43(12):1829-1840, 2017
- P481 Pre-existing cognitive dysfunction in critically ill patients and the incidence of delirium during ICU treatment.
- ST Könning, D Ramnarain Elisabeth Tweesteden Hospital Tilburg (ETZ), Tilburg, Netherlands Critical Care 2018, 22(Suppl 1):P481
- Rudolph JL, Marcantonio ER. Anesth Analg 12:1202-1211, 2001 P483 Validation of the SOS-PD scale for assessment of pediatric delirium: a multicenter study E Ista 1 , B Van Beusekom 1 , J Van Rosmalen 1 , MCJ Kneyber 2 , J Lemson 3 , A Brouwer 4 , G Dieleman 1 , B Dierckx 1 , M De Hoog 1 , D Tibboel 1 , M Van Dijk 1
- Erasmus MC -Sophia Children's Hospital, Rotterdam, Netherlands; 2 UMC Groningen -Beatrix Children's Hospital, Groningen, Netherlands;
- Radboud University Medical Center, Nijmegen, Netherlands; 4 Maastricht University Medical Centre, Maastricht, Netherlands Critical Care 2018, 22(Suppl 1):P483
- Wade et al. Crit Care 16:R192, 2012
- P486 Withdrawn P487 Withdrawn P488 Effect of acetaminophen on sublingual microcirculation in febrile septic patients: preliminary analysis U Falanga, R Domizi, E Casarotta, A Carsetti, S Tondi, A Donati Università Politecnica delle Marche, Ancona, Italy Critical Care 2018, 22(Suppl 1):P488
- Janz DR et al. Crit Care Med 43, 534-541, 2015 P489 Frequency, risk factors and symptomatology of iatrogenic withdrawal from opioids and benzodiazepines in critically ill neonates, children and adults: a systematic review of clinical trials MA Duceppe 1 , M Perreault 1 , AJ Frenette 2 , L Burry 3 , P Rico 2 , A Lavoie 4 , C Gélinas 5 , S Mehta 3 , M Dagenais 1 , D Williamson 2
- McGill University Health Centre, Montreal, Canada; 2 Hôpital du Sacré- Coeur de Montréal, Montreal, Canada; 3 Mount Sinai Hospital, Toronto, Canada; 4 CHU Ste-Justine, Montreal, Canada; 5 Jewish General Hospital, Montreal, Canada Critical Care 2018, 22(Suppl 1):P489
- Vincent JL et al. Crit Care 15:196, 2011 References
- Vuong C et al. Endocr Rev 31(1): 98-132, 2010.
- Lafuente A et al. Vet Hum Toxicol 36(6):524-8, 1994.
- Syvälahti E, Pynnönen S. Acta Pharmacol Toxicol (Copenh) 40(2):285-8, 1977. References
- Bomberg H et al.: Anaesthesia 69:1241-50, 2014.
- Romagnoli S et al: Crit Care Med. 45:e925-e931, 2017. P495 The use of intranasal fentanyl versus parenteral opioid for acute pain relief in adults: systematic review and meta-analysis F Fortier-Dumais, C Chau, JM Chauny, A Cournoyer, V Huard, J Lessard Hôpital Sacré-Coeur de Montréal, Montréal, Canada Critical Care 2018, 22(Suppl 1):P495
- Triantafillidis JK et al. World J Gastroenterol 19(4):463-81, 2013 P497 Sleep disorders in ICU survivors C Alexopoulou, A Proklou, M Fanaridis, S Soundoulounaki, M Bolaki, EM Antonogiannaki, E Kondili University Hospital of Heraklion, Crete, Heraklion, Greece Critical Care 2018, 22(Suppl 1):P497
- Thiboutot et al. Can J Hosp Pharm. 69(2):107-13, 2016 P500 Sedation practices and preferences of Turkish intensive care physicians: national survey S Urkmez 1 , E Erdogan 1 , T Utku 1 , Y Dikmen 1
- Reddy et al Continuing Education in Anaesthesia Critical Care & Pain, 12:140-144, 2012 References 1. SM. Jakob at al, JAMA. 2012
- MC. Reade at al, JAMA. 2016
- Delisle et al. Ann Intensive Care 1:42, 2011
- Pasin et al. PLoS ONE 8(12):e82913.
- Narisawa et al J Intensive Care 3:26, 2015 References
- Boumendil A et al. J Am Geriatric Soc 53:88-93, 2005 P514 Voolcano I: very old and old people with critical disease I E Sousa, T Leonor, M Fernandes, R Pinho Centro Hospitalar de Entre Douro e Vouga, Santa Maria da Feira, Portugal Critical Care 2018, 22(Suppl 1):P514
- Health Inequalities & People with Learning Disabilities in the UK: 2010 Emerson & Baines
- Death by Indifference. MENCAP 2004
- Confidential Inquiry into premature deaths of people with learning disabilities. CIPOLD 2013 P518 Comparison of home and clinic follow-up visits after hospital discharge for post-ICU patients: a cross-sectional study
- R Rosa 1 , C Robinson 1 , P Berto 2 , P Cardoso 2 , L Biason 3 , J Maccari 1 , D Sganzerla 1 , D Schneider 1 , P De Leon 1 , M Mattioni 1 , L Tagliari 1 , M Falavigna 1 , M Oliveira 1 , L Anzolin 1 , S Da Silva 1 , F Dutra 1 , S De Menezes 1 , D De Souza 1 , E Sanchez 1 , R Kochhann 1 , F Gomes 1 , G Medeiros 1 , V Machado 1 , J Andrade 1 , C Dietrich 1 , C Teixeira 1
- 1 Hospital Moinhos de Vento, Porto alegre, Brazil; 2 Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil; 3 Hospital Independência, Porto Alegre, Brazil Critical Care 2018, 22(Suppl 1):P518
- Witjes M et al. Am J Transplant 17(7):1922-7.
- P520 Physicians' ability to predict hospital mortality in acutely deteriorating patients: a cohort study J Ramos 1 , R Dias 2 , D Forte 2
- Hospital Sao Rafael, Salvador, Brazil; 2 Hospital das Clinicas, Sao Paulo, Brazil Critical Care 2018, 22(Suppl 1):P520
- Hoorn.S, et al. Crit Care:20, 2016. References
- Wall RJ, Engelberg RA, Downey L, Heyland DK, Curtis JR Crit Care Med 35:271-279, 2007
- P524 Breaking bad news in the emergency department: a randomized controlled study of a training using role-play simulation I Bragard 1 , JC Servotte 1 , I Van Cauwenberghe 2 , A Donneau 1 , N Dardenne 1 , R Tubes 1 , B Cardos 1 , M Guillaume 1 , A Ghuysen 1
- Université de Liège, Liège, Belgium; 2 University Hospital Centre of Liege, Liège, Belgium Critical Care 2018, 22(Suppl 1):P524
- Fallowfield L et al. The Lancet 363:312-319, 2004
- Bragard et al. Journal of Health Psychology 15(7):1075-1081, 2010
- Dosanjh et al. Medical Education 35:197-205, 2001
- Baile et al. The Oncologist 5:302-311, 2000
- Merckaert et al. British Journal of Cancer 109: 2507-2514, 2013 References
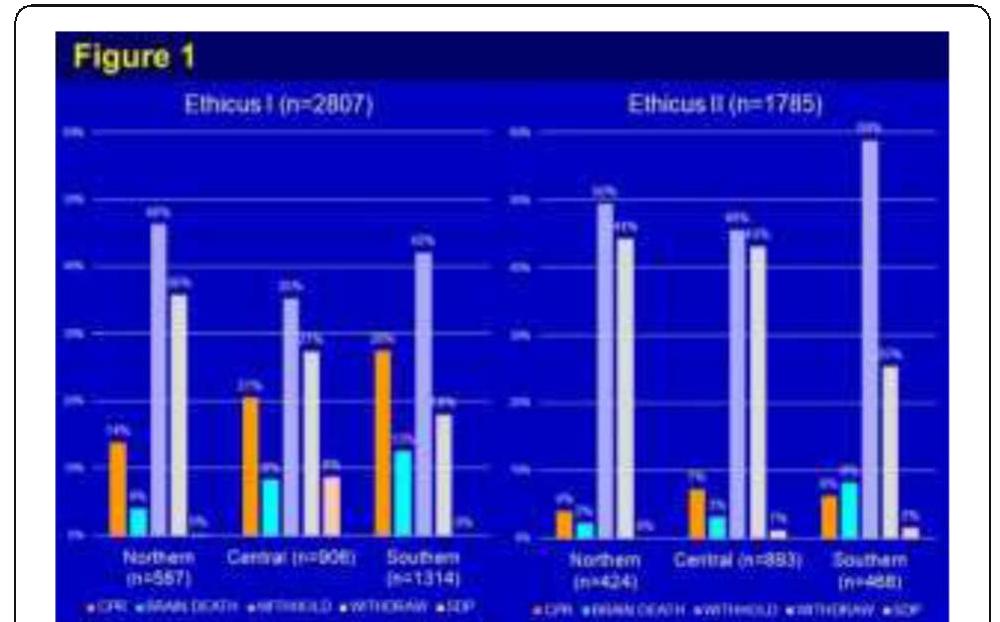
- Sprung CL et al. JAMA 2003; 290: 790-797 P528 Ethicus end-of-life practices (EOLP) in worldwide intensive care units (ICUs)-the ethicus II study A Avidan 1 , CL Sprung 1 , B Ricou 2 , A Michalsen 3 , C Feldman 4 , M Anstey 5 , M Baras 1 , M Weiss 6 , H Bulow 7 , C Hartog 8
- Hadassah Hebrew University Medical Center, Jerusalem, Israel; 2 Hôpital Cantonal Universitaire de Geneve, Geneve, Switzerland; 3 Tettnang Hospital, Tettnang, Germany; 4 University of the Witwatersrand, Johannesburg, South Africa; 5 Sir Charles Gairdner Hospital, Perth, Australia; 6 University Hospital Ulm, Ulm, Germany;
- Holbaek Hospital, Holbaek, Denmark; 8 University Hospital Jena, Jena, Germany Critical Care 2018, 22(Suppl 1):P528
- Mark, NM et al. ICM 2015;41:1572-1585
- Sprung CL et al. JAMA 290:790-797 P529 Multidisciplinary team perceptions about terminal extubation in a teaching hospital in Brazil S Ribeiro, M Fukuda, J Alencar, P Almeida, R Brandao-Neto Clinics Hospital, University of Sao Paulo Medical School, Sao Paulo, Brazil Critical Care 2018, 22(Suppl 1):P529 References
- Chotirmall SH, et al J Crit Care. 25(2):360. e361-e368, 2010 P530 Changing thoughts about end-of life care in the ICU; results of a survey M Kikuchi, A Yaguchi, M Kang, M Saito, M Tsunoda, T Oshiro, A Kim, K Shibahara, K Mochiduki, N Saito Tokyo Women's Medical University, Tokyo, Japan Critical Care 2018, 22(Suppl 1):P530 compared. Mann-Whitney U test was used for analysis. A p<0.05 was considered statistically significant. Results: There were total 51 participants (32 nurses and 19 doctors) in 2012 and 42 participants (23 nurses and 19 doctors) in 2017. The acceptances of withdraw therapy in nurses were significantly de- creased in 2017 than in 2012 (40% vs. 83%, 53% vs. 83%, p<0.05, respectively), while no changed in doctors. The positive of organ transplantation from brain death was also decreased in nurse in 2017 than in 2012 (43% vs. 80%, p<0.05).The feel guilty for withdraw therapy in nurses was also significantly decreased in 5 years (10% vs. 30%, p<0.05). Conclusions: Some of end-of-life thoughts in the ICU were shown differences in nurses compared with 5 years ago.
Related papers
Critical Care Medicine, 2000
Journal of applied physiology (Bethesda, Md. : 1985), 2002
Journal of Applied Pharmaceutical Science
Methods in Molecular Biology, 2016
Laboratory Investigation, 2012
Angiogenesis, 2010
Computer Methods and Programs in Biomedicine, 2000
Radiology, 2009
Investigative Ophthalmology & Visual Science, 2010
Microcirculation, 2003
Critical Care, 2018
International journal of biomedical imaging, 2006
Journal of Applied Physiology
Molecular Biomedicine
AJP: Lung Cellular and Molecular Physiology, 2006
Journal of Nuclear Medicine, 2013
Journal of Applied Physiology, 2003
 Bruno Pilote
Bruno Pilote
