Chemistry and Technology of Explosives
…
645 pages
1 file

Sign up for access to the world's latest research
AI-generated Abstract
This book serves as a comprehensive resource on the chemistry and technology of explosives, addressing both theoretical and practical aspects crucial for understanding the field. The content spans chemical, physical, and physico-chemical properties, as well as manufacturing processes, presented in a detailed and accessible format. This fourth edition expands upon previous versions, ensuring it meets the current needs of researchers and practitioners.
Figures (427)











![facts also confirm the existence of addition compounds of nitric acid and water.
Thus the refractive index shows, according to Veley and Manley [12], a linear
relation over the range from 78 to 91% concentration. At 91% a sharp inflection
occurs, and above 98.5% the slope of the curve is reversed. The electrical conduc-
tivity also shows anomalies over this concentration range, passing through a min-
imum.](https://figures.academia-assets.com/63254196/figure_002.jpg)




![Fic. 7. Ultra-violet absorption spectra of solutions of N,O; in anhy-
drous nitric acid. (R. N. Jones, Thorn, Lyne and E. G. Taylor [58)]).](https://figures.academia-assets.com/63254196/figure_007.jpg)

![THE RAMAN LINES OF NITRIC ACID AT DIFFERENT CONCENTRATIONS
various concentrations, while the line 1400 cm’ has not been observed.
Further investigations of Chédin [60a-62] and of Susz, Briner and Favarger [63]
have shown that a solution of nitric anhydride (NOs) in nitric acid produces both
1050 and 1400 cm’! lines. From this the assumption has been advanced that the
two lines indicate the presence of N.O; in mixtures of nitric and sulphuric acids.
Thus it seemed that Sapozhnikov’s theory (p, 10) had been confirmed. However.
further investigations have shown that this interpretation of the results is not
quite right. For Chédin stated that solutions of N,O; in carbon tetrachloride,
chloroform, nitromethane and phosphorus oxychloride produced the 707, 860,
1033, 1240 and 1335 cm’ lines (in addition to the solvent line), while there were](https://figures.academia-assets.com/63254196/table_011.jpg)




![Fic. 14. Viscosity of solutions HNO3-H,SO,-H,O. Change in viscosity with
increase of HNO; content at various temperatures (Swinarski and Piotrowski [52]).](https://figures.academia-assets.com/63254196/figure_013.jpg)








![Lauer and Oda [20] assumed the existence of nitracidium sulphate (according
to Hantzsch) and suggested that the mechanism of nitration with a nitrating mixture
is as follows:](https://figures.academia-assets.com/63254196/figure_022.jpg)
![- —_ SS = —
the addition mechanism.
Studies of the nitration of terpenes are of interest too, as they provide evidence
for the possibility of attaching a HNO; molecule to the double bond. Konovalov
[24] obtained nitro derivatives from menthene, camphene, pinene and bornylen¢
on acting with nitric acid. Bouveault [25] was able to prepare an addition produc
of camphene and HNO3. He obtained an oily product with a structure that could
10t be well defined. The reaction of addition of nitric acid to the double bond wa:
studied in detail by Sucharda [26]. He found that on acting on pinene with nitric
acid containing 33% of KNO; instead of with pure nitric acid, or by introducing
nitric acid vapours diluted with dry air, nitric acid esters were obtained in ove
10% yield. When reduced with zinc dust in the presence of ammonia, the esters
were converted to the corresponding alcohols.
aig ates](https://figures.academia-assets.com/63254196/figure_023.jpg)





![tensive investigations are those by Holleman [49-55], who in the period 1895-1924
carried out numerous experiments and systematized the data obtained.
Holleman [55] gives the following data on the composition of the nitration
products obtained in the nitration of different monosubstituted benzene deriva-
tives with mixtures of nitric and sulphuric acids (Table 2). As appears from the data
shown below, the substituent already present affects the orientation of the group
which is being introduced. It is evident that nitration can be influenced by the
steric factor. For exampl: tert.-butylbenzene is mainly nitrated in para (72.7%)
and to a much lesser extent in ortho (15.8%) positions (H. C. Brown and Nelson [88]).](https://figures.academia-assets.com/63254196/table_013.jpg)



![Vorozhtsov [21] referred to the nitration of m- nitroacetanilide (XIV) as a1
example of inconsistency between the results obtained and predicted, viz.:](https://figures.academia-assets.com/63254196/figure_031.jpg)






![It should be borne in mind that since the advent of chromatography, it is
now possible to separate and identify the constituents of complex mixtures
which formerly presented some difficulty. It therefore seems desirable that some
of the existing data on the composition of nitration products, particularly those
obtained in earlier studies should be re-examined using up to date techniques.
Finally attention must be drawn to the fact that the orienting effect of the nitro
group in nucleophile and radical reactions usually differs from that in electrophilic
reactions, and instead of meta orientation, ortho or para orientation takes place. The
corresponding observations are referred to in chapters dealing with nucleophile and
radical substitutions of nitro compounds (pp. 204, 207 and 212 respectively).
A monographic description of aromatic nitration and modern approach to
substitution rules was recently given by de la Mare and Ridd [78a].](https://figures.academia-assets.com/63254196/figure_036.jpg)
![Another scheme of Titov [32,39] suggests that the mechanism of oxidation
operates through the formation of an aryl nitrate, which is the result of attaching
NO," through the oxygen atom:
nitrating toluene. Since the phenolic group thus introduced then promotes the
introduction of the nitro groups, the number of the latter may be relatively large.
Thus, in the nitration of naphthalene to nitronaphthalene, 0.5-3.5% of 2,4-dinitro-
a—naphthol is formed (Fierz-David and Sponagel [80]). Titov [39] found dinitro-
phenol and picric acid in the products resulting from the nitration of benzene,
trinitro-m-cresol in the products of the nitration of toluene and trinitro-m-chloro-
phenol in the products of nitrating chlorobenzene.
Titov believes that phenols are formed from hydrocarbons under the influence](https://figures.academia-assets.com/63254196/figure_037.jpg)























![On the other hand Ingold and co-workers [144,155a] have proved that the
yresence of nitrous acid in the nitrating acid decreases the rate of nitration of
romatic compounds in general with the exception of phenols. The same holds
rue for phenyl ethers (e.g. anisole) which are more difficult to nitrate with higher
oncentrations of nitric acid in acetic acid (e.g. 8N) in the presence of nitrous
cid, whereas with a less concentrated nitric acid (e.g. 5N), nitrous acid accele-
ates the reaction.](https://figures.academia-assets.com/63254196/figure_058.jpg)


![Nitrous esters can react with alkyl peroxides to yield alkyl nitrates [163,221]
(see also Vol. ID).](https://figures.academia-assets.com/63254196/figure_061.jpg)
![Okon and Hermanowicz [181c] have found that the nitrate I can serve as a nitrat-
ing agent.](https://figures.academia-assets.com/63254196/figure_062.jpg)






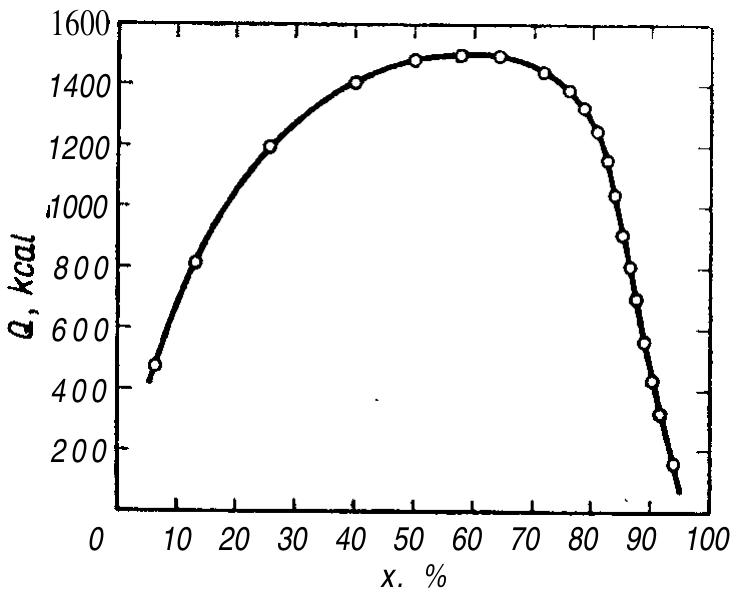
![Fic. 19. Heat evolved on mixing nitric acid with sulphuric acid
in relation to the water content A in these acids. Quantity of heat
Q =A B (Gelfman [5]).
be carried out at higher temperatures without any risk of exceeding the safety limit
and due to the higher temperature, nitration can proceed more rapidly.
Gelfman [5] has revised the generally accepted data for calculation of the heat
generated during mixing the acids and during their dilution with water [16]. He found
the absolute value of the heat generated in the reaction between sulphuric and
nitric acids to be lower in the presence of water than when the acids are in an an-
hydrous state. He also found it decreased on diluting the acids with water. This
relationship presented graphically is close to a linear one (Fig. 19). On the diagram](https://figures.academia-assets.com/63254196/figure_067.jpg)
![Fic. 21. Enthalpy of nitric acid, sulphuric acid, and water mixtures (McKinley
and Brown [6]).](https://figures.academia-assets.com/63254196/figure_069.jpg)
![Fic. 22. Specific heat of nitric acid, sulphuric acid, and water mixtures (McKinley
and Brown [6]).
at a temperature of 32°C. The total acid content in the mixture is 50%, and the HNO; content](https://figures.academia-assets.com/63254196/figure_070.jpg)
![3830-1840.5 = 1989.5 kcal or 19.9 kcal/kg
Fro. 23. Heats of dilution of nitric acid, sulphuric acid, and their
mixtures (Rhodes and Nelson [4]).](https://figures.academia-assets.com/63254196/figure_071.jpg)



![Fic. 26. Solubility of m- dinitrobenzene in sulphuric acid (Groggins et al. [1]).](https://figures.academia-assets.com/63254196/figure_075.jpg)

![Fic. 28. Rate of nitration of benzene to dinitrobenzene as a function of the molar
concentration of sulphuric acid. (Various curves correspond to various molar
concentrations of H,SO,, when [HNO;] = 1.) (Hetherington and Masson [9]).
Fic. 28. Rate of nitration of benzene to dinitrobenzene as a function of the molar](https://figures.academia-assets.com/63254196/figure_077.jpg)













![Fic. 40. Absorption spectra of nitroparaffins (1), and nitrodiols and _aliphitic
aminonitro compounds (II) (T. Urbanski [ll]).
Consequently the absorption curves of compounds I and II do not contain
maxima but only shoulders (Fig. 40). They are shifted towards longer wavelength
(bathochromic effect) as compared with the original nitroparaftlns.](https://figures.academia-assets.com/63254196/figure_090.jpg)


![ABSORPTION SPECTRA OF NITROBENZENE
Brand and his co-workers [17] carried out extensive studies on the absorption
spectra of aromatic compounds in sulphuric acid solutions, i.e. in a strongly proto-
nizing solvent. They found that under the influence of the sulphuric acid the
maximum of the nitro group shifted. These shifts were most pronounced in the
case of mononitro compounds, and the least in the case of trinitro compounds.
They were smaller when sulphuric acid was used as a solvent, and larger when
oleum was used. The absorption curves for 2,4-dinitrotoluene are shown in Fig.](https://figures.academia-assets.com/63254196/table_023.jpg)



![and Herrocks [43]. The last group of investigators determined the structure by
means of two-dimensional series and projections onto the principal planes. Be-
cause of limited accuracy attainable with the technique all that time the pattern
obtained was not clear enough. The molecule was not planar, the benzene ring
was distorted, and the N-O, bonds differed in length. It was only when Llewel-
lyn’s investigations [44] were published in 1947, in which a complete three-dimensional
Fourier synthesis was applied, that a definite pattern was obtained as shown in](https://figures.academia-assets.com/63254196/figure_095.jpg)
![first to carry out this research were Hertel [41], Bannerjee [42] and James, King](https://figures.academia-assets.com/63254196/figure_096.jpg)
![Fic. 46. Bond distances and angles of m- dinitrobenzene (Archer [45]).
Structures of the simplest compounds containing the nitro group, such as
for example, NO,, N,O,, HNO, and NO,", have already been discussed. The N-O
distances for nitromethane are 1.21 A and the bond angle 127° (Brockway, Beach
and Pauling [46] and Rogowski [47]).](https://figures.academia-assets.com/63254196/figure_097.jpg)
















![Aromatic hydroxylation is known to take place in animal and human organism
ind therefore it is of great importance to know the metabolism of various aro
matic compounds including drugs (D. Robinson, J. N. Smith, R.T. William
'47]). The presence of the nitro group in a molecule, resulting in its activatior
nay sometimes lead to a rather unusual course of reaction. The Richter [48] re
ction might be taken as an example, in which m- bromobenzoic acid may be obtaine
yy reacting potassium cyanide with p- nitrobromobenzene. Likewise, when reac
ng potassium cyanide with m- nitrobromobenzene, a mixture of o- and p- bromc
yenzoic acids are formed.
Accordins to Bunnett and his co-workers [49.50.50al the reaction is of th](https://figures.academia-assets.com/63254196/figure_115.jpg)
![Holleck and Perret [51] gave the following diagrammatic presentation of nucleo-
philic addition of the OH or CN ion to sym- trinitrobenzene in alkaline medium
(X=OH or CN):](https://figures.academia-assets.com/63254196/figure_116.jpg)



![Gitis believes that compounds of the V type are the main products of the Janovsky
reaction.
The formula V is not in agreement with the views expressed by various autho:
on the structure of the coloured products obtained by adding substances containin
an active methylene group to higher nitrated aromatic compounds, starting frot
m- dinitrobenzene. A number of papers have been published on the subject. They ori
ginated from the Jaffe-Folin [63,64] reaction for quantitative calorimetric determ
nation of creatinine. The reaction consists in the development of a red colot
when solutions containing creatinine are treated with aqueous picric acid and
few drops of alkali at room temperature. Many (but not all) compounds wit
active CH, group are capable of giving this reaction.
Several red compounds have been isolated from the red solution obtaine
ny + — . ae
The nature of the Janovsky’s colour reaction is not sufficiently understood
Reitzenstein and Stamm [55] were the first to try to establish the structure of the
compounds formed. They were able to isolate from an acetone solution a browr
product (IV), resulting from the reaction of 1,2,4-chlorodinitrobenzene with th
enolic form of acetone:](https://figures.academia-assets.com/63254196/figure_119.jpg)




![INHIBITION CONSTANTS IN THE POLYMERIZATION OF VINYL ACETATE AT 45°C
= dallateaiaaeal
Recently Inamoto and Simamura [74] investigated the interaction of I-cyano-
1-methylethyl radicals and various nitro compounds (nitrobenzene, m- dinitroben-
zene, nitromethane, tetranitromethane) and Bevington and Ghanem [84] have studied
the effects of picric acid and m- dinitrobenzene on the sensitized radical polymeri-
zation of styrene. Picric acid proved to be a rather inefficient inhibitor. m- di-
nitrobenzene was found to be a polymerization retardant. By using C-labelled
specimens of the nitro compounds the authors determined the amounts of nitro
sompounds incorporated in the polymer. The average number of retardant mole-
cules per polymer molecule was found to be 0.5-0.7.
On the basis of these experiments and of those of Inamoto and Sinamura,
Bevington and Ghanem suggest the interaction of polymer radical with m- dinitro-](https://figures.academia-assets.com/63254196/table_026.jpg)











![Ortho- and p- dinitrobenzene react with sodium sulphite toform the corre-
spending nitrosulphonic acids:
According to Golosenko (after Orlova [3]), m- dinitrobenzene reacts with sodium
sulphite at 70°C according to the scheme:](https://figures.academia-assets.com/63254196/figure_129.jpg)











![The problem was finally solved by Meisenheimer [40] in 1902, who found that
by the addition of CH;OK to trinitrobenzene an anisole derivative was formed:](https://figures.academia-assets.com/63254196/figure_136.jpg)


![Preparation from m- xylene. Giua [15] suggested sym-trinitrobenzene might
be prepared by the nitration of m- xylene to the trinitro derivative, followed by
oxidation and decarboxylation:](https://figures.academia-assets.com/63254196/figure_138.jpg)









![Fic. 54. Change of yield of MNT with
the ratio acid/toluene (Orlova [2a]).
Fic. 53. Influence of the rate of stirring
on the rate of nitration of toluene (Or-
en i Sy](https://figures.academia-assets.com/63254196/figure_147.jpg)

![Fic. 52. Nitration of toluene with HNO3-H,SO,-H,O mixture, at temperature,
of di- and trinitration 65° and 80°C respectively. Area between I and II - dinitration,
to the right of II - trinitration (Gorst [2]).
The distribution coefficient of HNO; between the toluene and acid layers is
0.066 at 5°C and the concentration of sulphuric acid 70% H,SO3. At lower acid
concentrations it is practically zero. This means that on heterogeneous nitration
nitric acid passes into the organic layer only in small quantities. Therefore there is
practically no nitration in this layer.](https://figures.academia-assets.com/63254196/figure_149.jpg)
![Fic. 55. change of the rate of nitration of
toluene with temperature (Orlova [2a]).
activity) on the rate of nitration of toluene:](https://figures.academia-assets.com/63254196/figure_150.jpg)

![SOLUBILITY OF TECHNICAL MNT (MIXTURE OF ISOMERS) IN SULPHURIC ACID (GORST [2])
THERMOCHEMICAL PROPERTIES
Garner and Abernethy [3] give the following thermochemical data for the
isomers of mononitrotoluene :](https://figures.academia-assets.com/63254196/table_042.jpg)








![SPECIFICATION FOR P-NITROTOLUENE (AFTER U.S.S.R. DATA, GorstT [2])](https://figures.academia-assets.com/63254196/table_048.jpg)
![SPECIFICATION FOR O- NITROTOLUENE (AFTER U.S.S.R. DATA, GORST [2])](https://figures.academia-assets.com/63254196/table_049.jpg)






![Fic. 59. Influence of temperature on the yield of DNT. Nitration of o- and p- nitroto-
luenes in nitrating mixtures with various concentrations of sulphuric acid (Kobe.
Skier and Prindle (({32]).](https://figures.academia-assets.com/63254196/figure_161.jpg)
![Fic. 60. Influence of the concentration of sulphuric acid on the yield of DNT.
Nitration of o- and p- nitrotoluenes (Kobe, Skinner and Prindle ([{32]).](https://figures.academia-assets.com/63254196/figure_162.jpg)




![3,6 (or 2,5-)-Dinitrotoluene was obtained by Page and Heasman [33] by the
oxidation of 5-nitro-o-toluidine with Caro’s acid.
All other isomers are prepared by nitration of m- nitrotoluene, followed by
fractional crystallization of the product.](https://figures.academia-assets.com/63254196/figure_167.jpg)






![Giua and Reggiani [87] reacted sodium alcoholate with trinitrotoluene in acetone
solution and obtained several addition products, containing various proportions of
alcoholate (1-3 molecules of C,H;ONa for 1 molecule of trinitrotoluene). By
treating the products with an inorganic acid, they obtained yellow, amorphous
compounds, which they regarded as mixture of several substances, which were
dibenzyl! delivatives. e.g.:](https://figures.academia-assets.com/63254196/figure_169.jpg)
![Stefanovich [88], on the basis of Meisenheimer’s formulae, ascribes the formulae
VII and VIII to the addition products of &—trinitrotoluene with two or three mole-
cules of potassium alcoholate respectively.](https://figures.academia-assets.com/63254196/figure_170.jpg)








![centration of sulphuric acid from 100 to 92% may be explained by the fact that
the concentration of HSO, ions increases considerably, whereas the concentration
of NO," ion decreases only slightly. Decreasing the concentration of sulphuric acid
below 92% H,SO, causes the NO,” concentration to fall more rapidly than the
HSO, concentration increases, thus decreasing the reaction rate.
To check their theory, Bennett and his co-workers added KHSO, to the nitrating
tion of dinitrotoluene to trinitrotoluene.
Orlova [110a] repeated the experiments of Bennett et al., studying the kinetic
of nitration of dinitrotoluene in nitrating mixtures rich in nitric acid that dissolve
dinitrotoluene, i.e. in homogeneous conditions. For 0.7 mole of dinitrotoluene
3.8 mole of HNO; in 12 mole of H,SO, were used. The concentration of sulphuric
acid was changed from 87 to 100% H,SO,. The temperature was 90°C. Contrary
to the results of Bennett, Orlova did not find any maximum of the rate of nitra
tion (Fig. 66) which she attributes to the homogeneity of the reaction medium.](https://figures.academia-assets.com/63254196/figure_178.jpg)
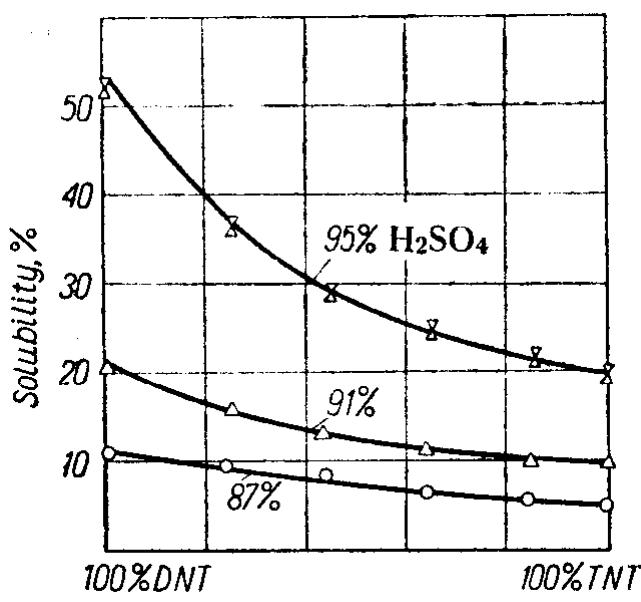






![Figure 72 shows the influence of trinitrotoluene on the rate of nitration of
linitrotoluene in heterogeneous systems at 90°C. It is interesting to note that the
iddition of 66-70% of trinitrotoluene to dinitrotoluene more than halves the
‘ate of the nitration, Further increase in the content of trinitrotoluene promotes
litration of dinitrotoluene. When the content of trinitro compound reaches 91%
he yield of trinitration is almost the same as that of the pure dinitrotoluene.
On the basis of her own experiments and those reported in the literature Orlova
[2a] came to the conclusion that the mechanism of nitration of toluene to TNT
n heterogeneous conditions can be depicted in the following terms.
Nitration of toluene to mononitrotoluene and of the latter to dinitrotoluene
ELD OF TRINITROTOLUENE WHEN MIXTURES OF DINITROTOLUENE](https://figures.academia-assets.com/63254196/table_061.jpg)


![The sensitiveness of TNT at 90°C is of the order of that of picric acid at room
temperature.
ee eee
In any case the handling of liquid TNT requires more safety measures than solid
TNT, though the fact that detonation in molten TNT proceeds only with great diffi-
culty reduces the danger. TNT becomes more sensitive when such solid substan-
ces as for example ammonium nitrate, are added to it (Vol. IV). Addition of
sulphur also increases the sensitiveness to impact (T. Urbanski and Pillich [93]).](https://figures.academia-assets.com/63254196/table_063.jpg)
![Fic. 74. Sensitiveness Of TNT to impact at various temperatures
(T. Urbanski and Sikorska [114]).](https://figures.academia-assets.com/63254196/figure_185.jpg)










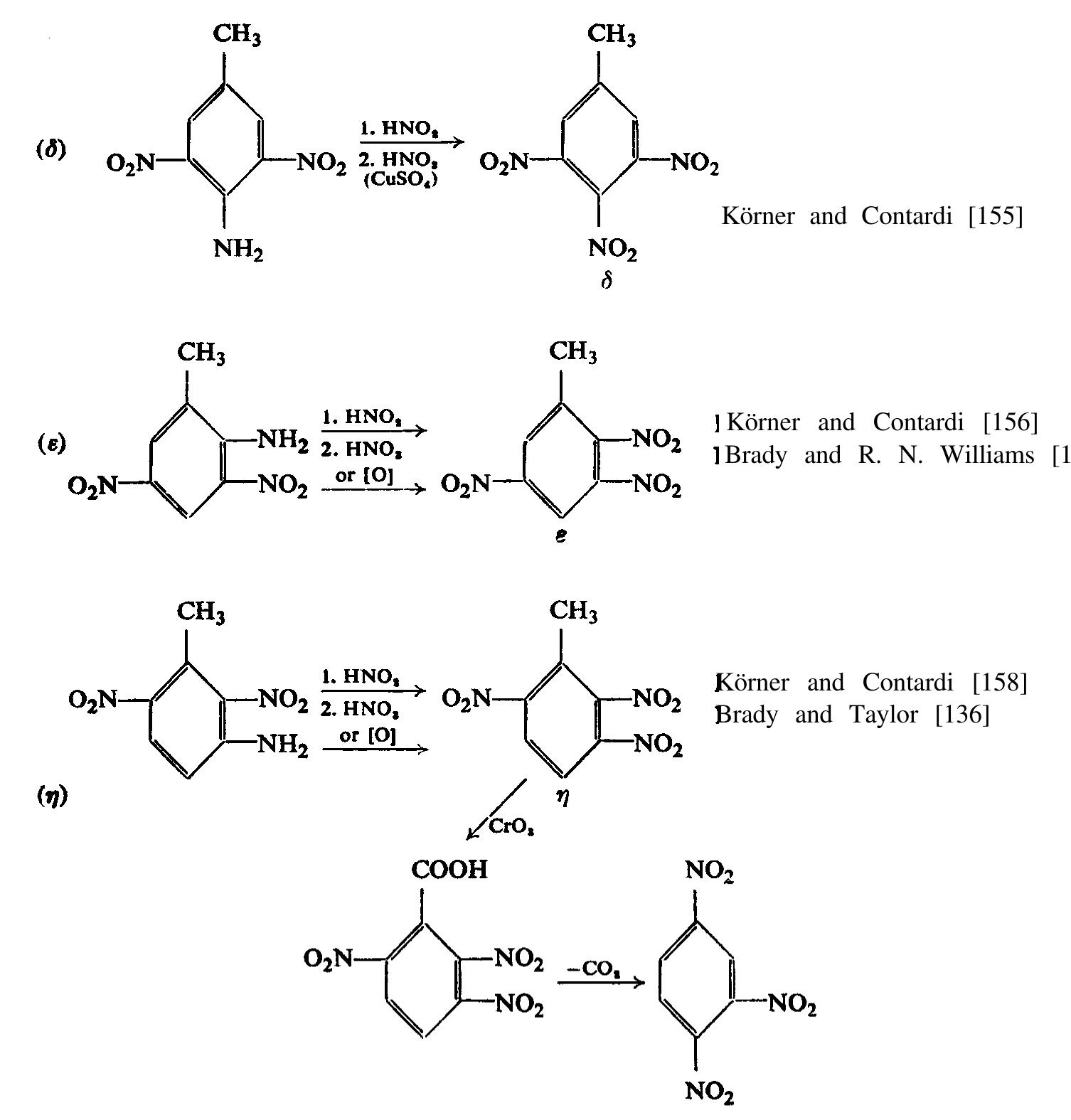
![The reaction was first mentioned by Laubenheimer [143] when examining
chloro-3,4-dinitrobenzene. However for a long time no notice was taken of the
possibility of putting it into practice. It was only during World War I that the
method was introduced in the U.S.A., and this happened quite accidentally. In
the search for methods of removing unsymmetrical isomers from crude TNT, the
reduction of trinitrotoluenes was studied. It was hoped that the nitro group in the
meta position, being chemically more active, would be more easily reduced, and
that the reduction product would be relatively soluble in water. Sodium polysulphide
was used for the reduction. However, it was found that the product of the reaction
was strongly contaminated with sulphur formed at the reaction. Among other re-
ducing agents used, sodium sulphite was shown to be a very efficient one in remov-
ing unsymmetrical isomers, its action consisting, however, not in the reduction
of the nitro group, but its replacement by a sulpho group.
As Muraour [103] found, the reaction of sodium sulphite was not confined to
a HE. LT a OE | ns. ge el 2 ee ryu?e. *¢&,—hl, Qe e eee es pecan BE Je](https://figures.academia-assets.com/63254196/figure_193.jpg)

![permanent and transient solubility of a-trinitrotoluene in solutions of sodium sul-
phite of different concentrations. The trend of the curves is similar to that found
earlier by Barbiére [145].](https://figures.academia-assets.com/63254196/figure_195.jpg)






![Fro. 80. Diagram of the lay-out of manufacture of TNT with detoluation [6].](https://figures.academia-assets.com/63254196/figure_203.jpg)
![FIG. 81. Flow sheet of the old method of manufacture of TNT in U.S.S.R. (Gorst ([7]).](https://figures.academia-assets.com/63254196/figure_204.jpg)





![In this plant each of the nitrators is connected with a separator, into which
the liquid from the nitrator overflows and where the nitro compound is separated
from the acid. The upper, nitro compound layer then flows to the next nitrator,
containing a more concentrated acid, while the lower acid layer passes through
a siphon to another nitrator, where less vigorous nitration takes place. Both liquid
phases-that of the acid and that of the material being nitrated-flow in counter-
current to each other. Figure 85 represents a schematic diagram of a unit for contin-
uous nitration (after MacNab [18]).](https://figures.academia-assets.com/63254196/figure_208.jpg)
![Fic. 86. Diagram of the German continuous nitration of toluene to TNT (CIOS XXIV 4).
are arranged in a cascade so as to enable the liquid to flow down from higher vessels
to lower ones. In this way the nitration mixture can be transferred from the nitra-
tor to the separator, where the nitro compound rises to the surface and flows off
through a drain between the separator to the next nitrator. The waste acid flows
down from the bottom of the separators to storage tanks.
The nitrators are 1.5 m high and 1 m in diameter. The separators are 0.75 m
high and 1.5 m in diameter. Both are fabricated from cast iron.
Recently F. Meissner, Wannschaff and Othmer [20] have published some data](https://figures.academia-assets.com/63254196/figure_209.jpg)





![Fic. 92. Continuous crystallization of TNT (Bofors-Norell method [26]).](https://figures.academia-assets.com/63254196/figure_215.jpg)




![Fic. 97. Sulphitation of TNT (British method [6]).](https://figures.academia-assets.com/63254196/figure_220.jpg)

![Fic. 100. Lay-out of a plant for continuous TNT manufacture according to Meissner [20].](https://figures.academia-assets.com/63254196/figure_222.jpg)
![Fic. 101 Lay-out of a Bofors plant for continuous TNT manufacture [17]. (Dimensions in meters).](https://figures.academia-assets.com/63254196/figure_223.jpg)
![British Technical Records [6] mentioned another approach to the problem of utili-
zation of sulphitation liquors. According to these data, attempts were made in Great
Britain during World War I to make use of the reactivity of the sulpho group in
the ortho or para position to the nitro ones. By acting with methylamine, N-methyl-
dinitrotoluidine (I and II) was obtained, which, when further nitrated, yielded
“methyltetryl”. All the methods mentioned proved uneconomical.](https://figures.academia-assets.com/63254196/figure_224.jpg)










![Later these observations were confirmed by Marqueyrol and Loriette [9]. From
100 parts of o- xylene, 130-135 parts of trinitro derivatives are obtained.
To separate the isomers use is made of their different solubility in 75%
ulphuric acid. The mixture of isomers is dissolved in the acid at 120-130°C, and
hen cooled; only the 3,4,5-isomer crystallizes then. The 3,4,6-isomer left in the acid
an be precipitated from the solution by adding water. The 3,4,5-isomer can be
yurified by crystallization from 75% sulphuric acid, the 3,4,6-isomer by
rystallization from alcohol.
Both isomers react with sodium sulphite to form the corresponding sulpho
ee eae](https://figures.academia-assets.com/63254196/figure_233.jpg)



![COMPARISON OF NITRATION CONDITIONS FOR TOLUENE AND XYLENE (PASCAL [20])
Mononitration of xylene (I. G. Leverkasen method). To the nitrator containing
1400 1. of spent acid from the previous nitration, 50 1. of xylene are added (thus the
230-300 parts of a nitrating mixture of the following composition are applied:](https://figures.academia-assets.com/63254196/table_072.jpg)
![Dinitromesitylene can be obtained by dissolving mesitylene in fuming nitric
acid, followed by the addition of water which causes dinitromesitylene to precip-
itate. For the preparation of trinitromesitylene by the Blanksma [7] method,
mesitylene is dissolved in sulphuric acid (partial sulphonation taking place), and
the solution is added to nitric acid (sp. gr. 1.52). Trinitromesitylene then precipi-
tates, as white crystals, dissolved by organic solvents only with difficulty. Kholevo
[26a] nitrated mesitylene with the nitrating mixture (27% HNO3, 69% H,SOu,
4% H,O) to yield trinitromesitylene.
The explosive power of trinitromesitylene is rather low - of the order of DNT.](https://figures.academia-assets.com/63254196/figure_236.jpg)

![Bonecki and T. Urbanski [45] prepared the same substance, also from 2,4,6-tri-
nitrotoluene, but in a different way:
The next steps were analogous to those mentioned earlier. High purity trinitrostyrene
was formed with m. p. 140°C.](https://figures.academia-assets.com/63254196/figure_238.jpg)







![It was known for long time that the 1,5- and 1,8-dinitronaphthalenes react
under the action of concentrated sulphuric acid to yield naphthazarine - a valuable
compound for dyeing [30]. The mechanism of the formation of this compound
(based on experiments of Dimroth and Ruck [18a]) probably consists in the trans-
formation of the nitro compounds to quinone-oximes and the reduction of one
of the nitro groups by hydroxylamine split off the oxime:](https://figures.academia-assets.com/63254196/figure_244.jpg)










![Fic. 108. Diagram of a nitrator for the nitration of naphthalene
(Pascal [20a]).
The mixture is prepared from 600 kg of the spent acid from the dinitronaph-
thalene manufacture and 550 kg of the spent acid from mononitration, which
has been re-used for washing dinitronaphthalene. In consequence the spent acid
contains some of the HNO; adsorbed by dinitronaphthalene. The composition and
the quantity of the mixture should be so calculated as to contain 128 kg of HNO3,
required for complete nitration of 300 kg of naphthalene.](https://figures.academia-assets.com/63254196/figure_254.jpg)


![NITRATION OF NITRONAPHTHALENE TO TRINITRONAPHTHALENE
French method [31a]](https://figures.academia-assets.com/63254196/table_076.jpg)




![When reacted with mercaptans or thiophenols of the general formula RSH
yields thioethers (Bielig and Reidies [23]):](https://figures.academia-assets.com/63254196/figure_258.jpg)












![Of these only the 2,4-isomer is used in explosive compositions or as a starting
material for the preparation of picric acid by one of the methods described later.
The 2,6-isomer on nitration also gives picric acid but it is not used for this purpose
on a commercial scale. Both these isomers may be obtained by the nitration of
phenol with nitric acid. All the other isomers are prepared by indirect methods.
Laurent [8] was the first to obtain dinitrophenol by nitrating phenol. Investi-
gations that followed revealed that Laurent’s dinitrophenol was not a chemical
individual, but a mixture of the 2,4- and 2,6-isomers. Kiirner [17] obtained pure
2,4-dinitrophenol by the nitration of p- nitrophenol and Armstrong [18] prepared
2,6-dinitrophenol along with some 2,4-isomer, starting from o- nitrophenol. Clemm
[19] determined the constitution of 2,4-dinitrophenol, which was later confirmed
by Salkowski [20].
Finally Hiibner and W. Schneider [21] defined the conditions under which the](https://figures.academia-assets.com/63254196/figure_270.jpg)






![SOLUBILITY OF PICRIC ACID IN WATER
The solubility of picric acid in sulphuric acid varies with the concentration
of the latter. It is highest for concentrated acid and lowest in 18-20% acid. This
can be seen from Table 110 [41b].](https://figures.academia-assets.com/63254196/table_082.jpg)


![SOLUBILITY OF PICRIC ACID IN NITRIC ACID (DRUCKER [42])
In organic solvents picric acid dissolves more readily than in water as Table
112 shows.](https://figures.academia-assets.com/63254196/table_085.jpg)
![SOLUBILITY OF PICRIC ACID IN ORGANIC SOLVENTS
The solubility of picric acid in aqueous solutions of methyl-, ethyl-, isopropyl-
and n-propyl alcohols, as well as of acetone has also been determined (Duff and
Bills [43]), Table 113.](https://figures.academia-assets.com/63254196/table_086.jpg)




![According to Ishiwara [71], after a 30 min action a 0.04% aqueous solution o:
picric acid exhibits bactericidal activity against typhoid bacteria, staphylococci,
streptococci and gonococci.](https://figures.academia-assets.com/63254196/figure_277.jpg)









![Fic. 116. Flow sheet of the nitration of sulphophenol to picric acid
(Pascal [2]).](https://figures.academia-assets.com/63254196/figure_286.jpg)
![Fic. 117. General view of nitrators for the production of picric acid [8].](https://figures.academia-assets.com/63254196/figure_287.jpg)

![FIG. 120. Flow sheet of the nitration of phenol with concentrated nitrating mixture (Lebedev [5]).](https://figures.academia-assets.com/63254196/figure_289.jpg)


![c. 123. Flow sheet of the production of picric acid from chlorobenzene (Lebedev [5]).](https://figures.academia-assets.com/63254196/figure_292.jpg)









![Hemmelmayer [28] presented the nitration of resorcylic acid and its subsequent
lecarboxylation by the following reactions :](https://figures.academia-assets.com/63254196/figure_298.jpg)
![Sulphonation yields only three compounds: I, II, and III
The sulphonic acids I and II can be obtained by sulphonation of resorcinol with
sulphuric acid or oleum at temperatures which are not higher than 100°C (Mertz
and Zetter [32]). The trisulphonic acid can only be prepared by the action of oleum
at 200°C. According to Aubertein and Emeury [29], resorcinol can be sulphonated
to the compound II by the action of a tenfold quantity (by weight) of sulphuric
acid of concentration 92-97.5% H,SO, or oleum (105% H,SO,) at 50°C. However,
a small proportion (1%) of resorcinol remains unchanged and is subjected to oxi-
dation during the subsequent nitration. It is responsible for foaming during the
nitration. According to the above authors, prolonged sulphonation or application
of more concentrated oleum does not prevent the presence of unsulphonated resor-
cinol.](https://figures.academia-assets.com/63254196/figure_299.jpg)
![3,5-Dinitropyrocatechol (m. p. 164°C) was prepared by Nietzki and Moll [38]
by nitrating pyrocatechol diacetate with cold cont. nitric acid, followed by
hydrolysis of ester groups with sulphuric acid.](https://figures.academia-assets.com/63254196/figure_300.jpg)



![A mixture of the tetranitroanisole isomers may be obtained (according to Claes-
sen [7]) by the nitration of m- nitroanisole. The compounds are not stable since their
nitro groups in the meta position are readily hydrolysed or substituted (van Duin
and van Lennep [8]). Their sensitiveness to impact is similar to that of TNT. The
expansion they give in a lead block is about 135% of that given by TNT.](https://figures.academia-assets.com/63254196/figure_304.jpg)




![(G. M. Robinson and R. Robinson [13], Gosh [14], Heertjes, Dahmen and
Wierda [ 11).](https://figures.academia-assets.com/63254196/figure_309.jpg)

![2,2’ ,4,4’,6,6’-Hexanitrodiphenyl sulphone (m. p. 307°C, decomposition) for
yellowish crystals difficult to dissolve in most organic solvents.
In 1912 Sprengstoff A. G. Carbonit [17] was granted a patent for a meth
of preparation of this explosive, consisting in reacting hexanitrodiphenyl sulph
with nitric acid. Since picryl chloride, as the starting material for picryl sulphi
was rather expensive, another method of preparation of hexanitrodiphenyl sulpho
via tetranitrodiphenyl sulphide, was also used. The latter was obtained by treat:
shlorodinitrobenzene with sodium thiosulphate. Then it was nitrated and oxidiz
SImuItaneously with nitric acid to hexanitrodinhenv|] sulnhone:](https://figures.academia-assets.com/63254196/figure_311.jpg)



![Although, according to Fliirscheim, tetranitroaniline has sufficient thermal
stability, even a product of the highest purity does not give a satisfactory heat test.
According to Ingraham [19b], tetranitroaniline has shown evidence of decom-
position by the heat test at 65.5°C when only a small amount of moisture was pie-
sent. The main product of decompositions was II. Prolonged heating at 75°C
results in loss of a nitro group. At 120°C decomposition takes place which proceeds
in a way similar to that of tetryl. The initiation temperature is 231-233°C. The
specific gravity of the product is 1.867 [19].
With regard to explosive power and sensitiveness to impact tetranitroaniline
1 a ee ae 2 A @ <a cee rs r 1](https://figures.academia-assets.com/63254196/figure_315.jpg)
![2,2’ ,4,4’,6,6’-Hexanitrodiphenylamine (m. p. 243-245°C) was first mentioned
in the chemical literature in 1876 and as long ago as 1891 Haussermann [21] drew
attention to its explosive properties. The product is known under the names of
Dipicrylamine, Hexyl, Heksyl, Hexamite, Hexamin, etc.
The annlicatian af heyy] ac an exynlacive anec hack ac far ace 1011 Tt wae widely](https://figures.academia-assets.com/63254196/figure_316.jpg)

![In addition to the 1,3,6,8-isomer a product, named by Ciamician and Silbe
the Y— isomer, has been obtained, which, according to recent investigations, he
proved to be the 1,2,6,8-isomer (II). From the reaction product pure compoun
I may be isolated by extraction with toluene, followed by crystallization from acet
acid. The pure product I may also be obtained by treating the crude product wit
sodium sulphite. According to Murphy, Schwartz, Picard and Kaufman [34], tt
melting point of the product may be raised in this way from 278° to 296°C ;
the cost of a 10% loss of yield. The same authors found that during the nitratic
process the 1,2,6,8-isomer (ID) is formed along with the principal product. Thi
isomer may be obtained in a larger quantity if carbzole is subjected to sulphons
tion with oleum prior to nitration.
The constitution of tetranitrocarhazole (1) was determined by Borsche an](https://figures.academia-assets.com/63254196/figure_318.jpg)



![The use of hexanitro-oxanilide (m. p. 295-300°C) as an explosive material was
suggested by the Societe Anonyme d’Explosifs [39].](https://figures.academia-assets.com/63254196/figure_322.jpg)



![Tetranitromethane like polynitro-aromatic hydrocarbons is able to form additior
compounds (p. 222, Fig. 47). Nevertheless, the existence of an addition compound
of tetranitromethane with benzene has not been proved by thermal analysis, when
this was carried out recently by T. Urbanski, Piskorz, Centner and Maciejewski
59]. They also examined a number of other systems by means of thermal analysis.
[he compositions of various eutectics determined by the above authors are shown
in Table 127.](https://figures.academia-assets.com/63254196/figure_326.jpg)
![TABLE 128
The explosive properties of nitrobenzene-tetranitromethane solutions were
examined in detail by Roth [62] who measured rates of detonation power (on
a 10.5 by 7 mm crusher gauge), and sensitiveness to impact, using nitroglycerine
and TNT as standards (Table 129). Lead block expansions are not included here
as they were not determined by standard methods.](https://figures.academia-assets.com/63254196/table_096.jpg)




Related papers
TEAM, 2018
Explosives are an integral part of the mining services whether it is a mines, stone quarries, infrastructure development works, tunnelling etc. In this paper a review has
iv I hereby declare that all information in this document has been obtained and presented in accordance with academic rules and ethical conduct. I also declare that, as required by these rules and conduct, I have fully cited and referenced all material and results that are not original to this work. Name, Last name : Serhat VARIŞ Signature : v ABSTRACT MOLECULAR MODELLING OF SOME EXPLOSIVES AND PROPELLANTS VARIŞ, Serhat
2000
The first successful syntheses of inorganic compounds by the shock wave loading of mixtures of substrates were reported in [1] and . Zinc ferrite was obtained from a mixture of oxides ZnO and Fe 2 O 3 . Titanium carbide was produced by the loading of titanium and carbon mixtures. Further investigations have shown that many various compounds can be synthesized in this way. The most important of them are listed in . A broader list can be found in Batsanov's review paper .
Breckenridge, CO. The descriptive text has not changed. The Wall Chart has been corrected and updated with chemical symbols of the explosives. An Appendix of Engineering Tools has been added. There is a need in the pyrotechnic, explosive, and propellant engineering and scientific community to compile the energetic material property and characteristic data for a single point reference. The objective of this paper is to fulfill that need for the properties and characteristics of selected high explosives of interest to the defense and aerospace industry. The information is collected from published literature and compiled for easy access in data sheet and wall chart format. Members of the engineering and scientific community of all disciplines are invited for input to the development of the knowledge base that is represented. Equally important to presenting the data is to identify the source as reference, which is listed at the end of this paper. This paper is updated periodically to include recent changes. Explosives referenced in MIL-STD-1316 are discussed together with common secondary explosives:
Journal of Chemical and Engineering Data, 1963
Synthesis of n e w analogs of tetryl and the determination of explosive properties are given. The correlation between the explosive properties assigned by the explosive sensitivity to impact, to friction, the explosive power and brisance, and the molecular structure represented by modified oxygen balance is graphically shown.
2009
Fire, explosion and chemical incidents most frequently occur either in chemical plants, military warehouses or during the transportation of dangerous and toxic substances, nevertheless they might appear in terrorist attacks, demolitions or sabotages. In this paper we shall present an instance of an explosion that occurred in the military warehouse near Paraćin. The explosion was caused by inadequate storage of the ammunition that was out of use, thus it can be referred to as hazardous waste.
Journal of visualized experiments : JoVE, 2016
Developmental testing of high explosives for military applications involves small-scale formulation, safety testing, and finally detonation performance tests to verify theoretical calculations. small-scale For newly developed formulations, the process begins with small-scale mixes, thermal testing, and impact and friction sensitivity. Only then do subsequent larger scale formulations proceed to detonation testing, which will be covered in this paper. Recent advances in characterization techniques have led to unparalleled precision in the characterization of early-time evolution of detonations. The new technique of photo-Doppler velocimetry (PDV) for the measurement of detonation pressure and velocity will be shared and compared with traditional fiber-optic detonation velocity and plate-dent calculation of detonation pressure. In particular, the role of aluminum in explosive formulations will be discussed. Recent developments led to the development of explosive formulations that resu...
1996
At the Department of Energy (DOE) and Sandia National Laboratories, a major effort has existed to develop new pyrotechnic formulations and explosive materials that have improved safety and sensitivity properties during use and handling. The driving force for these development efforts has been enhanced personnel safety plus improved safety/ sensitivity properties of these materials and their applications in regards to nuclear weapon safety issues. These efforts have produced a series of pyrotechnic and explosive materials that can replace traditional primary explosives such as lead styphnate and lead azide. This development has resulted in new pyrotechnic formulations and explosive materials that are insensitive to initiation by electrostatic discharge from a human body. Other safety properties have also been improved. The electrostatic insensitive pyrotechnics are a family of titanium subhydride (TiH x , X greater than 0.65)/potassium perchlorate (KClO 4) formulations. These titanium subhydride/potassium perchlorate pyrotechnics also have high temperature stability, high impact and friction insensitivity. The new explosive materials are inorganic coordination compounds based upon 5substituted tetrazolato pentaammine cobalt (III) perchlorates. Substituents in the tetrazole ring that have proven deflagration-to-detonation (DDT) properties include the cyano (-CN), nitro (-NO 2) and chloro (-Cl) groups. Their explosive properties include human body electrostatic insensitivity and high temperature stability along with friction and impact properties similar to RDX and HMX. The properties and uses of these materials has resulted in proven, mature technologies for the Department of Energy, Department of Defense as well as commercial, private sector applications.

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
Related papers
Proceedings of the National Academy of Sciences, 2006
ANNALS OF THE ORADEA UNIVERSITY. Fascicle of Management and Technological Engineering., 2010
Advances in Materials Science and Engineering, 2012
Clinical Approaches and Procedures in Cosmetic Dermatology, 2018
Central European Journal of Energetic Materials, 2011
Zavarivanje i zavarene konstrukcije, 2014
Management Systems in Production Engineering, 2015
Energies, 2020
Journal of Chemical Technology and Metallurgy, 2020
 Мари Михайлова
Мари Михайлова
