An analyticai procedure involving Sequential chemical extractions has been developed for the partitioning of Particulate trace metals (Cd, Co, Cu, Ni, Pb, Zn, Fe, and Mn) into five fractions: exchangeable, bound to carbonates, bound to... more
Anti-DPPH radical effect as well as anti-peroxide activity of o-hydroxyl, o-methoxy, and alkyl ester derivatives of p-hydroxybenzoic acid in a bulk fish oil system and its O/W emulsion were investigated. Electronic phenomena, intra-and/or... more
The CASTEP code for first principles electronic structure calculations will be described. A brief, nontechnical overview will be given and some of the features and capabilities highlighted. Some features which are unique to CASTEP will be... more
Highly Enantioselective Organocatalytic Addition of Unmodified Aldehydes to N-Boc Protected Imines: One-Pot Asymmetric Synthesis of β-Amino Acids. -With L-proline as the catalyst, β-amino aldehyde derivatives are obtained in up to 99%... more
This study examines the evidence for the effectiveness of active learning. It defines the common forms of active learning most relevant for engineering faculty and critically examines the core element of each method. It is found that... more
Concern about environmental protection has increased over the years from a global viewpoint. To date, the prevalence of adsorption separation in the environmental chemistry remains an aesthetic attention and consideration abroad the... more
We present a statistical model to estimate the accuracy of peptide assignments to tandem mass (MS/MS) spectra made by database search applications such as SEQUEST. Employing the expectation maximization algorithm, the analysis learns to... more
A statistical model is presented for computing probabilities that proteins are present in a sample on the basis of peptides assigned to tandem mass (MS/MS) spectra acquired from a proteolytic digest of the sample. Peptides that correspond... more
To obtain a semi-theoretical equation for the excess Gibbs energy of a liquid mixture, Guggenheim's quasi-chemical analysis is generalized through introduction of the local area fraction as the primary concentration variable. The... more
New, hydrophobic ionic liquids with low melting points (<-30°C to ambient temperature) have been synthesized and investigated, based on 1,3-dialkyl imidazolium cations and hydrophobic anions. Other imidazolium molten salts with... more
Metabolite profiling in biomarker discovery, enzyme substrate assignment, drug activity/specificity determination, and basic metabolic research requires new data preprocessing approaches to correlate specific metabolites to their... more
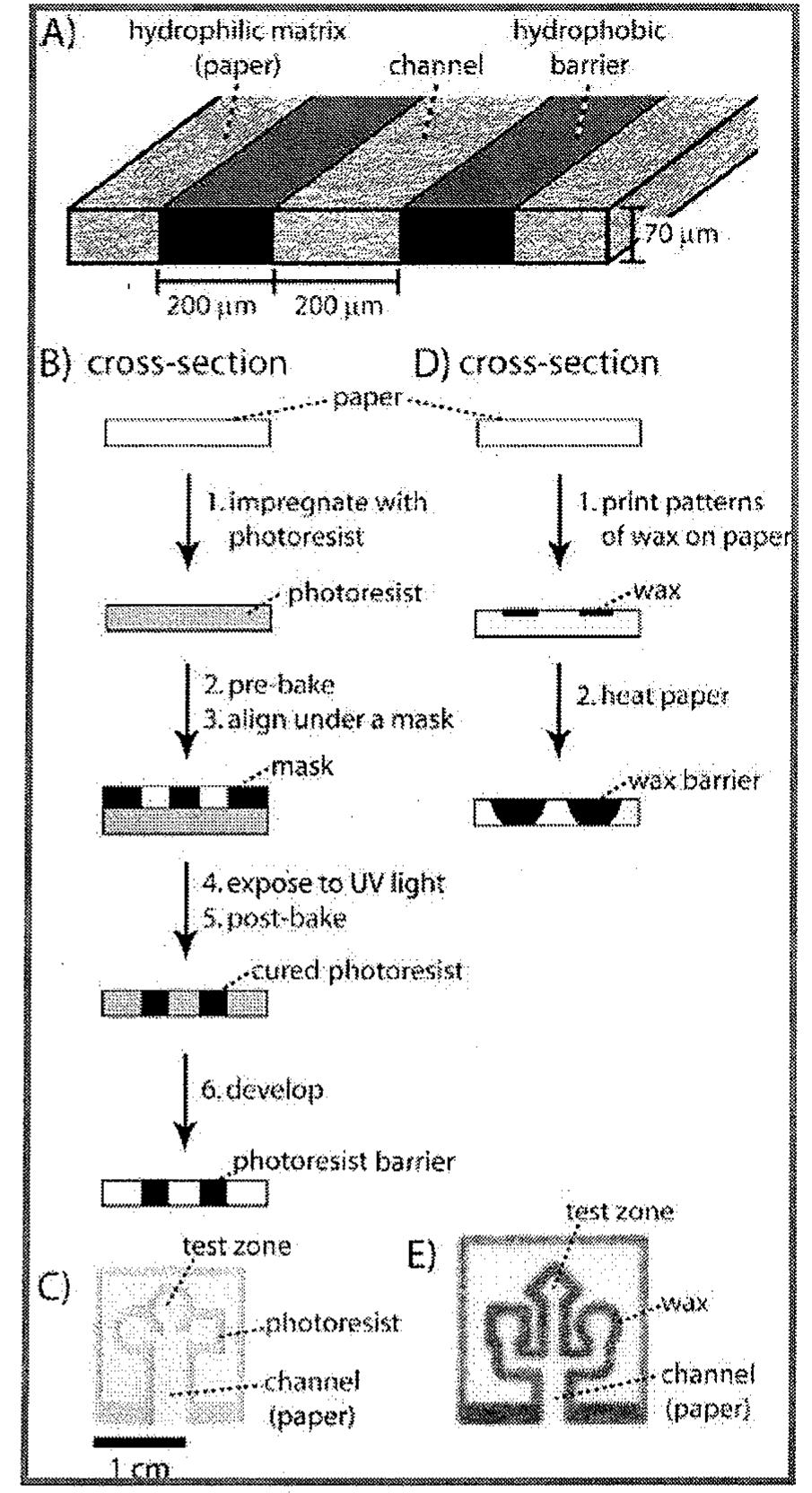
Microfluidic devices are finding increasing application as analytical systems, biomedical devices, tools for chemistry and biochemistry, and systems for fundamental research. Conventional methods of fabricating microfluidic devices have... more
Automobile manufacturers and fuel cell developers have produced PEMFCs for many years, but recent significant technological advances have left two major remaining challenges to widespread fuel cell use: cost and lifetime, which are... more
Oxide nanofluids were produced and their thermal conductivities were measured by a transient hot-wire method. The experimental results show that these nanofluids, containing a small amount of nanoparticles, have substantially higher... more
Being a motion on a discontinuity set of a dynamic system, sliding mode is used to keep accurately a given constraint and features theoretically-infinite-frequency switching. Standard sliding modes provide for finite-time convergence,... more
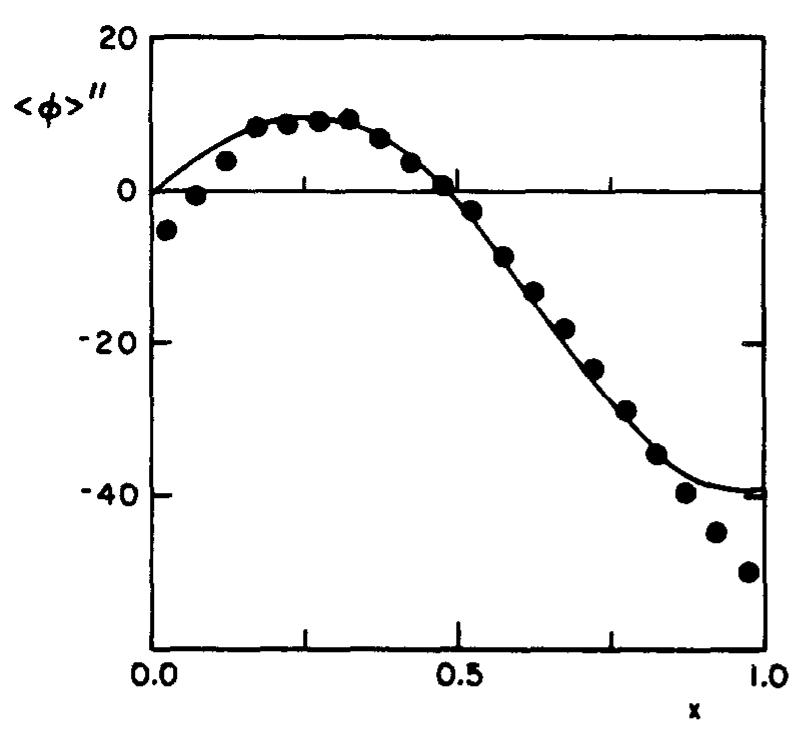
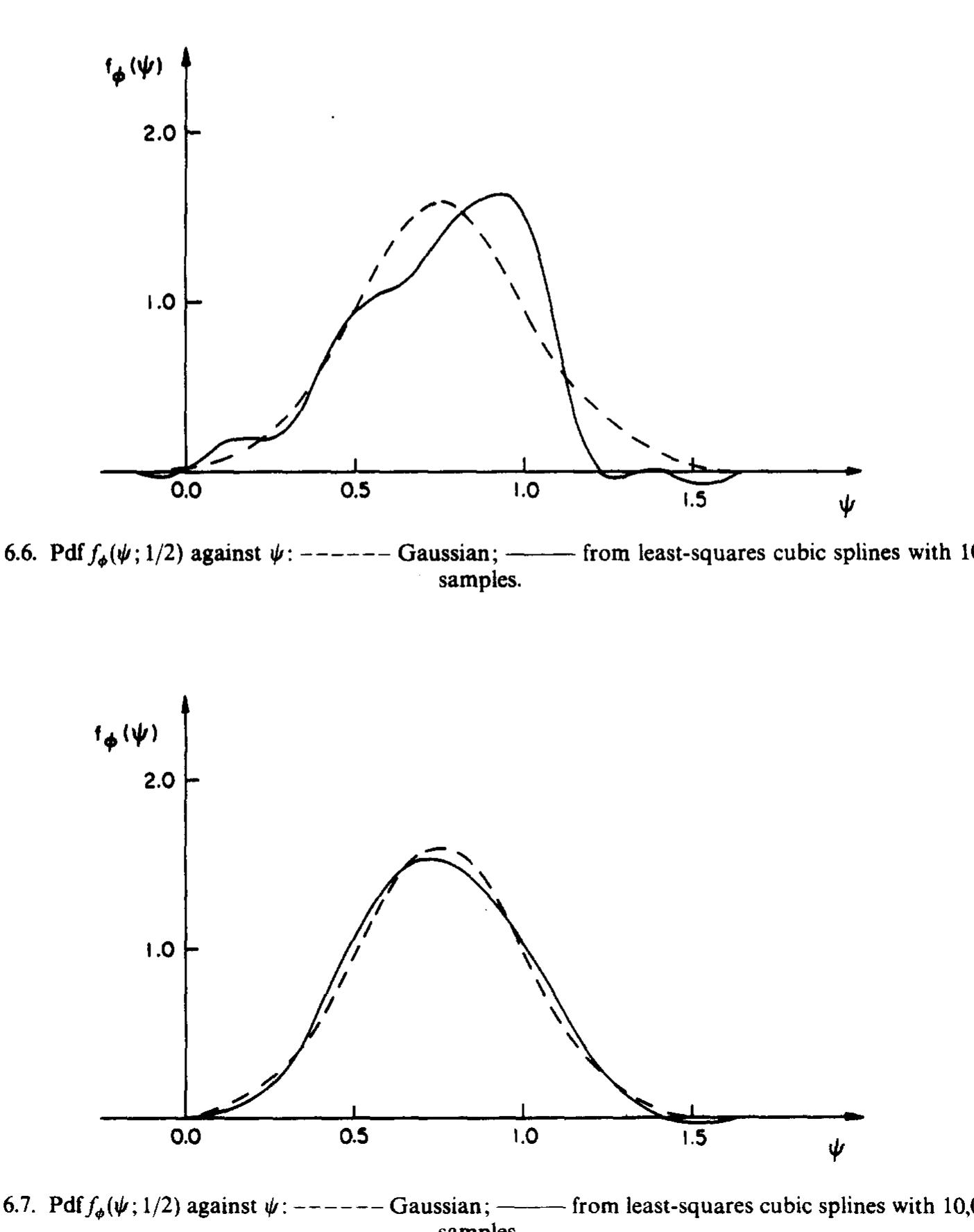
The aim of the methods described is to calculate the properties of turbulent reactive flow fields. At each point in the flow field, a complete statistical description of the state of the fluid is provided by the velocity-composition joint... more
Novel gold catalysts were prepared by coprecipitation from an aqueous solution of HAuCl 4 and the nitrates of various transition metals. Calcination of the coprecipitates in air at 400 °C produced ultrafine gold particles smaller than 10... more
We use multifractal detrended fluctuation analysis (MF-DFA), to See query 1 study sunspot number fluctuations. The result of the MF-DFA shows that there are three crossover timescales in the fluctuation function. We discuss how the... more
In this final part, we discuss fault diagnosis methods that are based on historic process knowledge. We also compare and evaluate the various methodologies reviewed in this series in terms of the set of desirable characteristics we... more
Research and development activities directed toward commercial production of cellulosic ethanol have created the opportunity to dramatically increase the transformation of lignin to value-added products. Here, we highlight recent advances... more
We describe a modification of two-dimensional (2-D) polyacrylamide gel electrophoresis that requires only a single gel to reproducibly detect differences between two protein samples. This was accomplished by fluorescently tagging the two... more
Most of the particle number emitted by engines is in the nanoparticle range, D (50 nm, while most of the mass is in the accumulation mode, 50 nm(D (1000 nm, range. Nanoparticles are typically hydrocarbons or sulfate and form by nucleation... more
The original protocol of two-dimensional electrophoresis with immobilized pH gradient (IPG-Dalt; Görg et al., Electrophoresis 1988, 9, 531±546) is updated. Merits and limits of different methods for sample solubilization, sample... more
The quantitative experimental uncertainty in the structure of fully hydrated, biologically relevant, fluid (L K ) phase lipid bilayers has been too large to provide a firm base for applications or for comparison with simulations. Many... more
Biodiesel is an alternative diesel fuel that is produced from vegetable oils and animal fats. It consists of the monoalkyl esters formed by a catalyzed reaction of the triglycerides in the oil or fat with a simple monohydric alcohol. The... more
Information is stored in neural circuits through long-lasting changes in synaptic strengths 1,2. Most studies of information storage have focused on mechanisms such as long-term potentiation and depression (LTP and LTD), in which synaptic... more
In contrary to other 1D momentum-conserving lattices such as the Fermi-Pasta-Ulam β (FPU-β) lattice, the 1D coupled rotator lattice is a notable exception which conserves total momentum while exhibits normal heat conduction behavior. The... more
Modern analytical tools should enable highly specific identification and characterization of inorganic and organic matter with minimal effort for sample preparation. Raman spectroscopy is one such highly specific method that enables... more
The TiO 2 /UV photocatalytic degradation of methylene blue (MB) has been investigated in aqueous heterogeneous suspensions. In addition to a prompt removal of the color, TiO 2 /UV-based photocatalysis was simultaneously able to oxidize... more
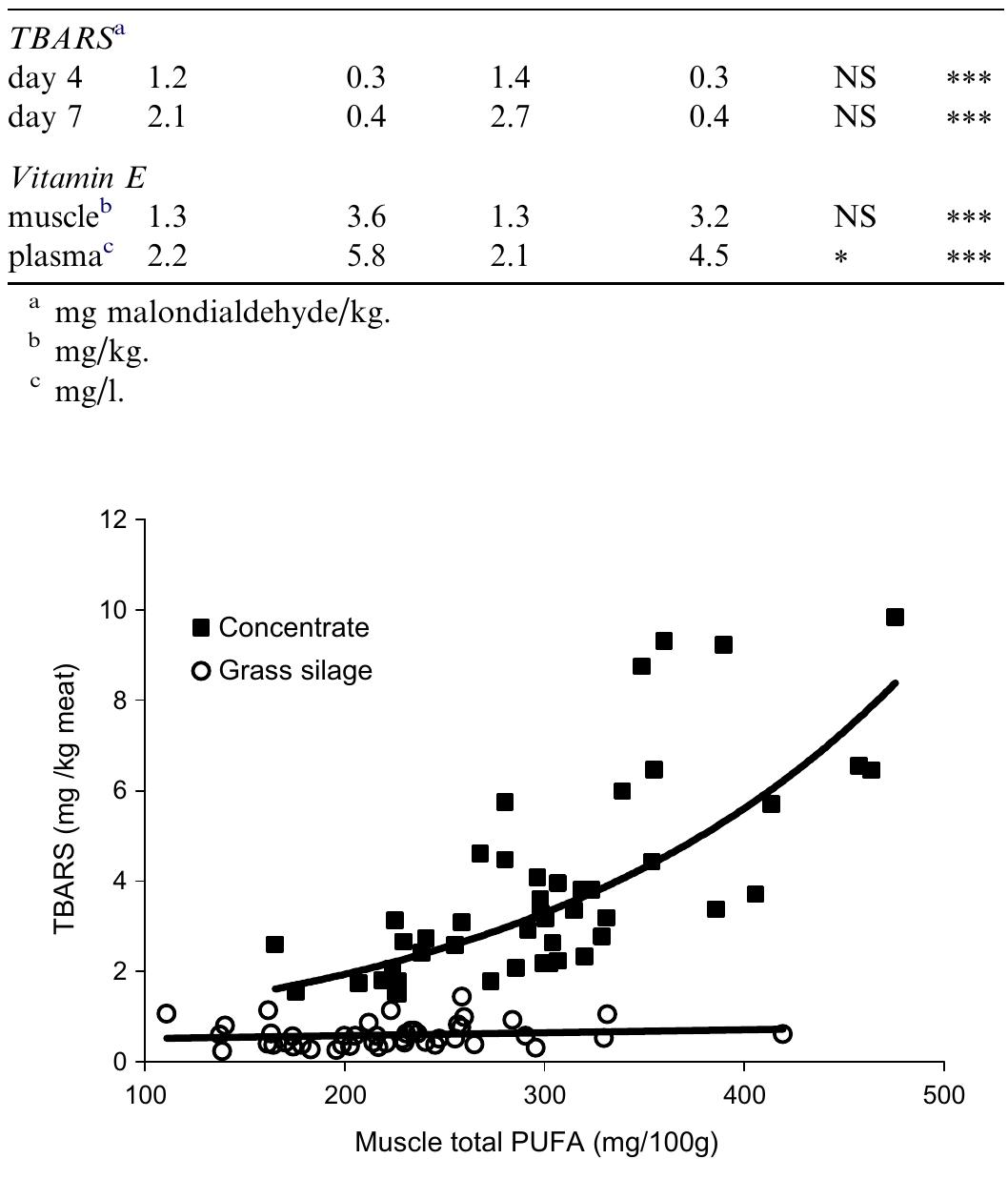
Interest in meat fatty acid composition stems mainly from the need to find ways to produce healthier meat, i.e. with a higher ratio of polyunsaturated (PUFA) to saturated fatty acids and a more favourable balance between n-6 and n-3 PUFA.... more
Simplified reaction mechanisms for the oxidation of hydrocarbon fuels have been examined using a numerical laminar flame model. The types of mechanisms studied include one and two global reaction steps as well as quasi-global mechanisms.... more
Over the past decade, the formation and growth of nanometer-size atmospheric aerosol particles have been observed at a number of sites around the world. Measurements of particle formation have been performed on di erent platforms (ground,... more
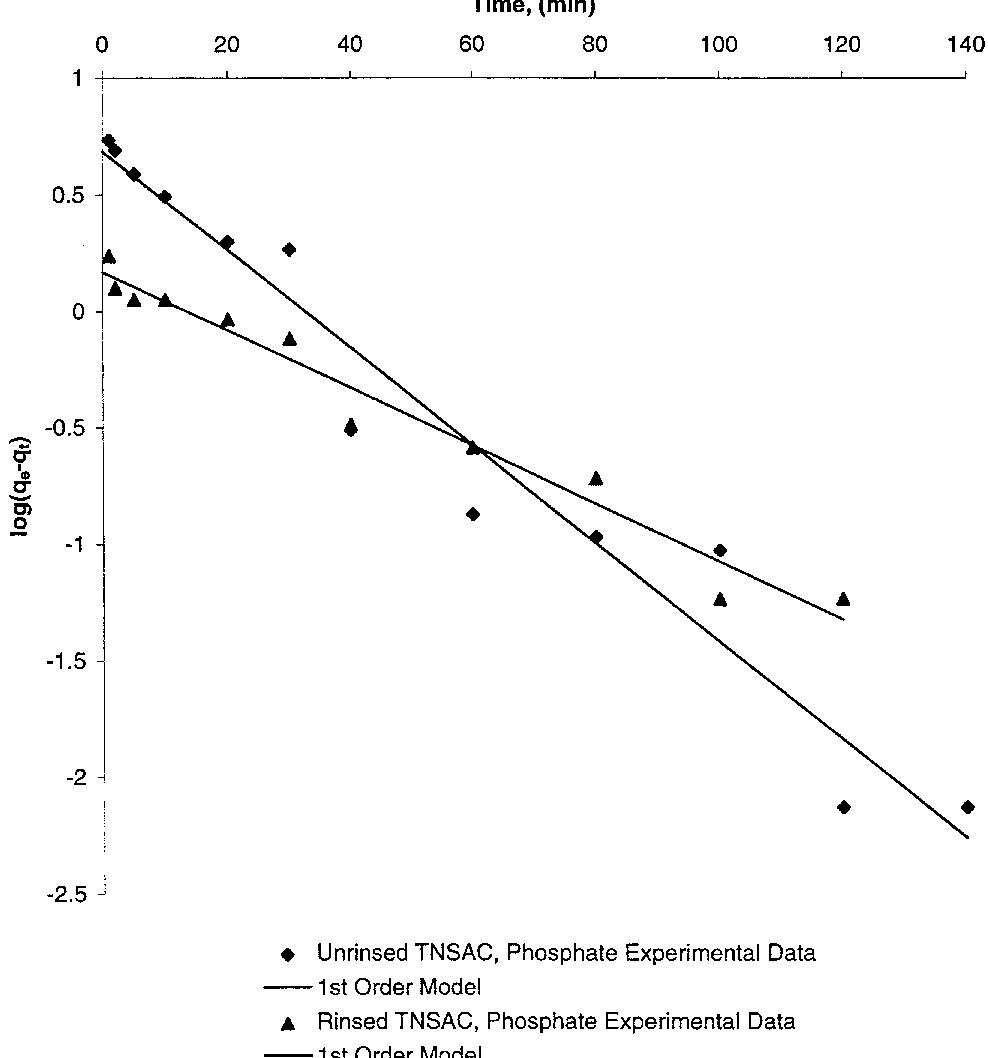
A com parison of kinetic models describing the sorption of pollutants has been reviewed. The rate m odels evaluated include the Elovich equation, the pseudo-® rst order equation and the pseudo-second order equation. Results show that... more
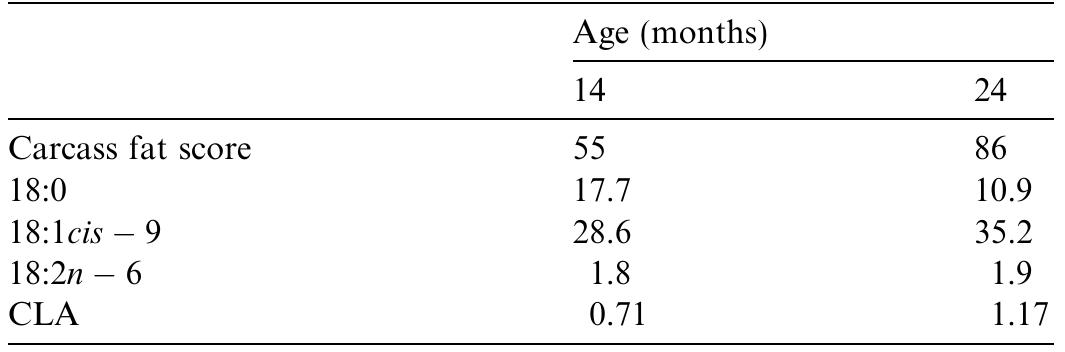
This paper reviews the factors affecting the fatty acid composition of adipose tissue and muscle in pigs, sheep and cattle and shows that a major factor is the total amount of fat. The effects of fatty acid composition on meat quality are... more



















![Table 3. Effect sizes for academic achievement associated with various aspects of problem-based learning. Beyond producing positive student attitudes, the effects of PBL are less generally accepted, though other supporting data do exist. Vernon and Blake [19], for example, present evidence that there is a statistically significant improvement of PBL on students’ clinical performance with an effect size of 0.28. However, Colliver [22] points out that this is influenced strongly by one outlying study with a positive effect size of 2.11, which skews the data. There is also evi- dence that PBL improves the long-term retention of knowledge compared to traditional instruction [51-53]. Evidence also suggests that PBL promotes better study habits among students. As one might expect from an approach that requires more independence from students, PBL has frequently been shown to increase library](https://figures.academia-assets.com/35815824/figure_003.jpg)
![Table 1. Collaborative vs. individualistic learning: Reported effect size of the improvement in different learning outcome: The importance of student engagement is widely accepted and there is considerable evidence to support the effectiveness of student engagement on a broad range of learning outcomes. Astin [38] What do these results mean in real terms instead of effect sizes, which are sometimes difficult to interpret? With respect to academic achievement, the lowest of the three studies cited would move a](https://figures.academia-assets.com/35815824/table_001.jpg)
![Lists of adsorption isotherms models. energy distribution, with most of its sites has an adsorption energy lower than the peak (maximum) or mean value [23]. Table 1 in homogeneous or heterogeneous systems due to its versatility [22]. Typically, a minimization procedure is adopted in solving the equations by maximizing the correlation coefficient between the experimental data points and theoretical model predictions with solver add-in function of the Microsoft excel [26]. In the limit, it approaches Freundlich isotherm model at high concentration (as the exponent # tends to zero) and is in accordance with the low concentration limit of the ideal Langmuir condition (as the 6 values are all close to one) [67].](https://figures.academia-assets.com/34054642/table_001.jpg)










![Fig. 1. Type of biodegradable nanoparticles: According to the structural organiza- tion biodegradable nanoparticles are classified as nanocapsule, and nanosphere. The drug molecules are either entrapped inside or adsorbed on the surface. (Originally adapted from [39] but modified.)](https://figures.academia-assets.com/51115310/figure_001.jpg)
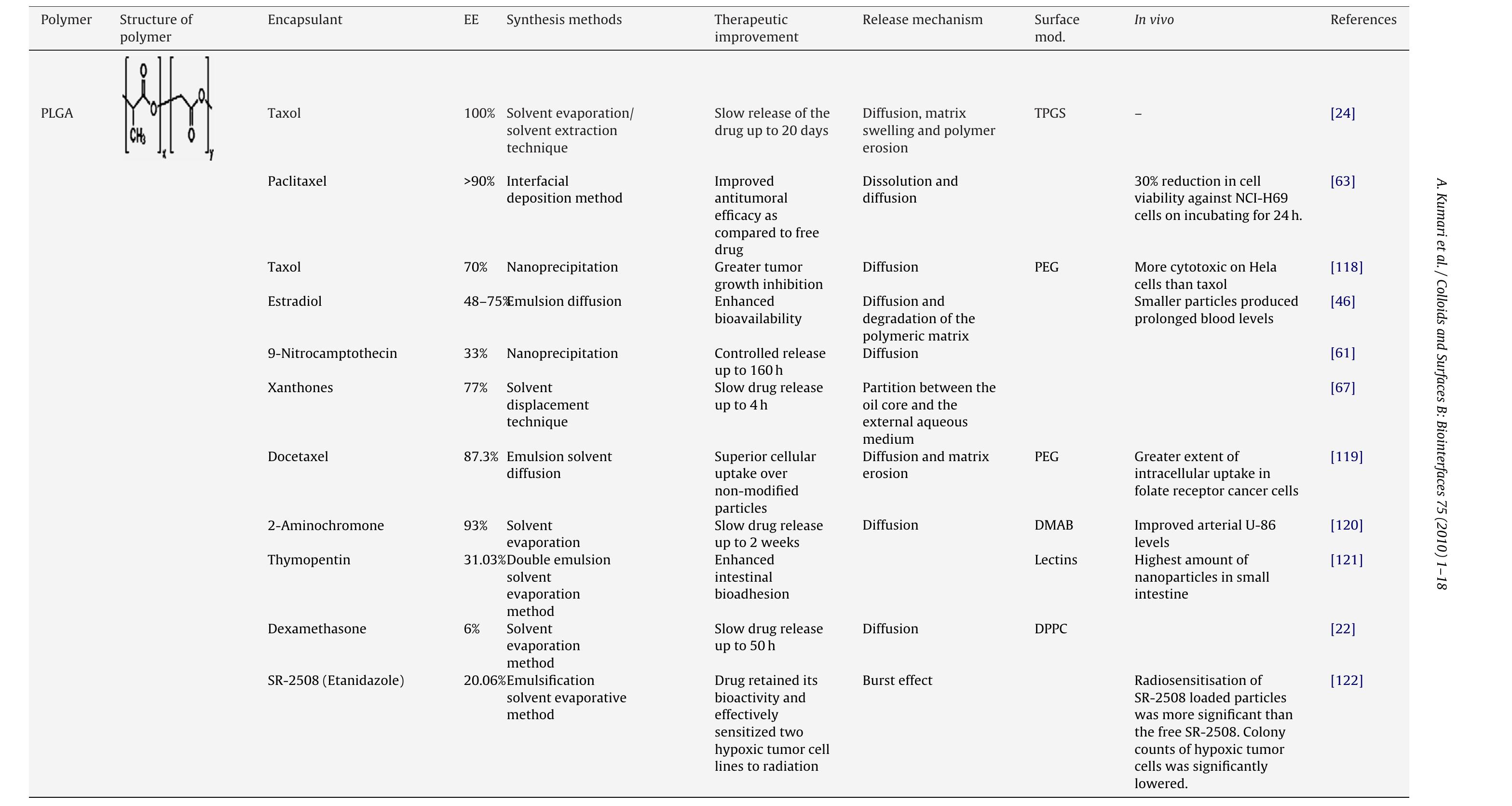
![Fig. 2. Hydrolysis of PLGA nanoparticles: PLGA nanoparticles are biologically hydrolyzed in acidic medium into lactic and glycolic acid. These hydrolysis products have been metabolized in TCA cycle. PLGA nanoparticles have been mostly prepared by emulsification—-diffusion [46], solvent emulsion-evaporation [36], interfacial deposition [42] and nanoprecipitation method [47] (Fig. 3). Generally in emulsification-diffusion method, the PLGA polymers are dissolved in organic solvent (EtAc, MEK, PC, BA, etc.), poured and separated in aqueous phase having stabilizer and subsequently emulsified by homogenizer. In solvent evaporation method, the polymers are dissolved in volatile organic solvent (DCM, acetone, CHCl3, EtAc, etc.) and poured into continuously stirring aqueous phase with or without emulsifier/stabilizer and sonicated. Interfacial deposition methods have been used for the formation of both nanocapsule and nanospheres. The nanoparticles are synthesized in the interfacial layer of water and organic solvent (water miscible) and finally the nanoparticles are Polymeric nanoparticles have been synthesized using various methods [42] according to needs of its application and type of drugs to be encapsulated. These nanoparticles are extensively used for the nanoencapsulation of various useful bioactive molecules and medicinal drugs to develop nanomedicine. Biodegradable polymeric nanoparticles are highly preferred because they show promise in drug delivery system. Such nanoparticles provide controlled/sustained release property, subcellular size and bio- compatibility with tissue and cells [43]. Apart from this, these nanomedicines are stable in blood, non-toxic, nonthrombogenic, nonimmunogenic, noninflammatory, do not activate neutrophils, biodegradable, avoid reticuloendothelial system and applicable to various molecules such as drugs, proteins, peptides, or nucleic acids [11]. The general synthesis and encapsulation of biodegradable nanomedicines are represented in Fig. 1. The drug molecules either bound to surface as nanosphere or encapsulated inside as nanocap- sules.](https://figures.academia-assets.com/51115310/figure_002.jpg)


































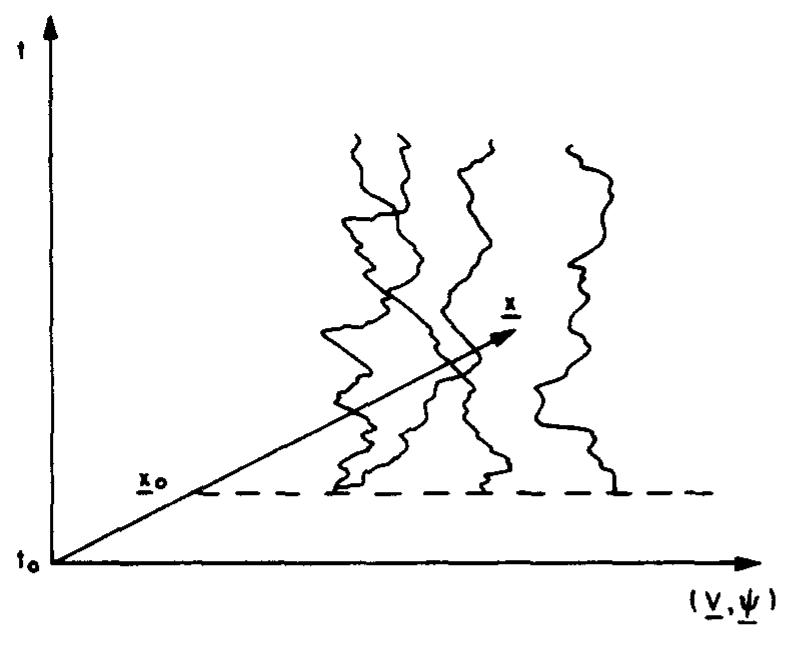





![Fic. 4.7. Sample of the Weiner process W,. ts < ty, [W,, —W,,] and [W,, — W,,] are independent Gaussian random variables with zero means and variances (t, —t,) and (t, — 1) respectively.+ The increment](https://figures.academia-assets.com/50465736/figure_018.jpg)




















































![and T, is the collector stagnation temperature, i.e. the temperature of the absorbing plate when the flow rate is equal to zero, and is obtained from: In addition to serving as a heat trap by admitting shortwave solar radiation and retaining longwave thermal radiation, the glazing also reduces heat loss by convection. The insulating effect of the glazing is enhanced by the use of several sheets of glass, or glass plus plastic. The top loss coefficient in Eq. (22) is given by [98]:](https://figures.academia-assets.com/2610411/figure_014.jpg)





















![Fig. 36. Detail schematic of a solar water heating system. Heat pumps use mechanical energy to transfer thermal energy from a source at a lower temperature to a sink at a higher temperature. Electrically driven heat pump heating systems have two advantages compared to electric resist- ance heating or expensive fuels. The heat pump’s COP is high enough to yield 11 to 15 MJ of heat for each kW h of energy supplied to the compressor [21], which saves on purchase of energy, and usefulness for air conditioning in the summer. Water-to-air heat pumps, which use solar heated water from the storage tank as the evaporator energy source, are an alternative auxiliary heat source. Use of water A load heat exchanger is shown in Fig. 36 to transfer energy from the tank to the air in the heated spaces. The load heat exchanger must be adequately designed to avoid excessive temperature drop and corresponding increase in the tank and collector temperatures.](https://figures.academia-assets.com/2610411/figure_036.jpg)















![Sequence of quality tests for solar collectors [109]](https://figures.academia-assets.com/2610411/table_009.jpg)

![Fig. 6. A speculative attempt to indicate possible interrelationships between phosphatidylinosito] breakdown and other receptor-linked phenomena which occur at cell surfaces. This figure should be viewed only as a very tentative attempt to visually portray the suggestions presented in the text. PI, phosphatidylinositol; 1,2-DG, 1!,2-diacylglycerol.](https://figures.academia-assets.com/49931424/figure_007.jpg)

![THE EFFECTS OF STIMULI ON THE LABELLING AND CONCENTRATION OF PHOSPHATIDYLINOSITOL AND ON TH LING OF SOME PRECURSOR POOLS IN VARIOUS TISSUES The effects of stimulation are summarised relative to the degree of stimulation seen with incorporation of P; into PI. -+, stimulus of similar magn slight +-, stimulus apparent, but much lower than with P;; n.c., no change; —, decreases. If no symbol is given, then no study was available. Nuc, n tides; 3GP, 3-glycerophosphate; [PI], concentration of phosphatidylinositol; Ino, inositol; Gly, glycerol; Glu, glucose; FA, fatty acid; Ac, acetate.](https://figures.academia-assets.com/49931424/table_002.jpg)
![EFFECTS OF ADRENERGIC STIMULATION AND BLOCKADE ON PHOSPHOLIPID TURNOVER IN VARIOUS TISSUES 5-hydroxytryptamine do, however, need precise identification. In the case of glucose acting on islets of Langerhans uncertainty exists as to whether a cell surface receptor is involved at all. These cells contain a plasma membrane glucoreceptor [281] which may be involved in controlling insulin release but other evidence points to immediate control by an intracellular metabolite of glucose [282]. No attempt to resolve this question for the recently-described phosphatidylinositol response [226] has yet been reported.](https://figures.academia-assets.com/49931424/table_003.jpg)

![EFFECTS OF CYCLIC AMP AND RELATED COMPOUNDS ON PHOSPHOLIPID LABELLING wT WE PREVA R g NER PEAS PV EAUEREUIOLUENE LEEW RCEUIUEEO EEE VY UOENE Ue be ole The first experiments of this type demonstrated that the changes in phospholipid metabolism in liver and parotid gland which adrenergic stimuli evoked could not be reproduced by dibutyryl cyclic AMP [213,218]. A similar pattern has now become established for several systems: in no situation does cyclic AMP, dibutyryl cyclic AMP or theophylline (which raises intracellular cyclic AMP concentrations by inhibiting cyclic AMP phosphodiesterase) mimic the effects of stimuli on phosphati- dylinositol metabolism (Table V). The only system which appeared briefly to be an exception was the thyroid, in which thyrotropin (TSH) evokes a spectrum of responses which includes enhanced phosphatidylinositol turnover; here it was claimed that the effect on phosphatidylinositol metabolism was duplicated by dibutyryl cyclic AMP {285a]. However, subsequent work has not substantiated this and theophylline does not elicit a phosphatidylinositol response (Table V). It seems likely that the reported effect could be attributed to an unspecific increase in phospholipid labelling which was secondary to a increased influx of 3?P, into tissue slices treated with the cyclic AMP derivative [241].](https://figures.academia-assets.com/49931424/table_005.jpg)
![TIME OF EARLIEST REPORTED OBSERVATION OF CHANGED PHOSPHATIDYLINO- SITOL OR PHOSPHATIDIC ACID METABOLISM IN STIMULATED CELLS synaptic plasma membranes during this time), and about half of the phosphatidylino- sitol lost from acetylcholine-treated pancreas in | h [20,193]. If this breakdown is localised to cellular sites such as plasma membranes and related membranes, which may initially contain a relatively small proportion of the phosphatidylinositol of the cell, the effects of such breakdown on the phosphatidylinositol concentration at these sites may be substantial before a gross change in the phosphatidylinositol content (or radioactivity) of the whole cell is apparent, i.e. within seconds or a very few min of the application of the stimulus. TABLE VI](https://figures.academia-assets.com/49931424/table_006.jpg)
![VIB. The time-course and the responsiveness to agonists The conclusion that enhanced phosphatidylinositol turnover, measured as stimulated labelling, could not be related closely to other responses of stimulated cells because it did not show either an earlier or parallel kinetic curve, but occurred somewhat later [183,187], was based on the implicit assumption that the time-course of phosphatidylinositol labelling gave a reasonable kinetic reflection of the then unknown first responsive reaction. It now appears that this first step, phospha-](https://figures.academia-assets.com/49931424/table_007.jpg)
![in higher plants (rice grains [41] and cress seedlings (Michell and Hawthorne, un-](https://figures.academia-assets.com/49931424/figure_001.jpg)



![Fig. 4. A suggested model for the events in lipid metabolism in a cell exposed to a stimulus which produces enhanced phosphatidylinositol turnover (slightly modified from Lapetina and Michell [155]). PI, phosphatidylinositol; PA, phosphatidic acid.](https://figures.academia-assets.com/49931424/figure_005.jpg)
![VID. Phosphatidylinositol breakdown as a reaction involved in receptor mechanisms If the model in Fig. 5b is accepted as a basis for discussion then two immediate predictions can be made. First, phosphatidylinositol cleavage must not only be localised at the plasma membrane, as suggested in Section IV F, but during activation it must also be physically coupled to cell-surface receptors. Second, an enhanced rate of phosphatidylinositol cleavage (and turnover) must occur whenever it can be shown that a particular type of receptor (e.g. muscarinic cholinergic or a-adrenergic) is present and functioning ina cell. Neither of these predictions are essential to model (a) of Fig. 5, and attempts to test them may help to distinguish between the alternative models. There is some evidence which suggests that the link between the receptor and — ee eee Eee —e eee eee ee eee eee ee ee There is some evidence which suggests that the link between the receptor and phosphatidylinositol breakdown cannot be a simple matter of control of an enzyme by the activated receptor. The strongest comes from experiments of Hokin [20] which showed that acetylcholine-stimulated phosphatidylinositol breakdown in the pancreas is prevented by dinitrophenol and therefore it presumably requires input of metabolic energy. Our own attempts to study this point in the parotid using two other inhibitors (CN7~ and oligomycin) have given less conclusive results (Jones, L. M. and Michell, R. H., unpublished data) but they have also suggested some role of metabolic enerev in the mechanism of the breakdown.](https://figures.academia-assets.com/49931424/figure_006.jpg)

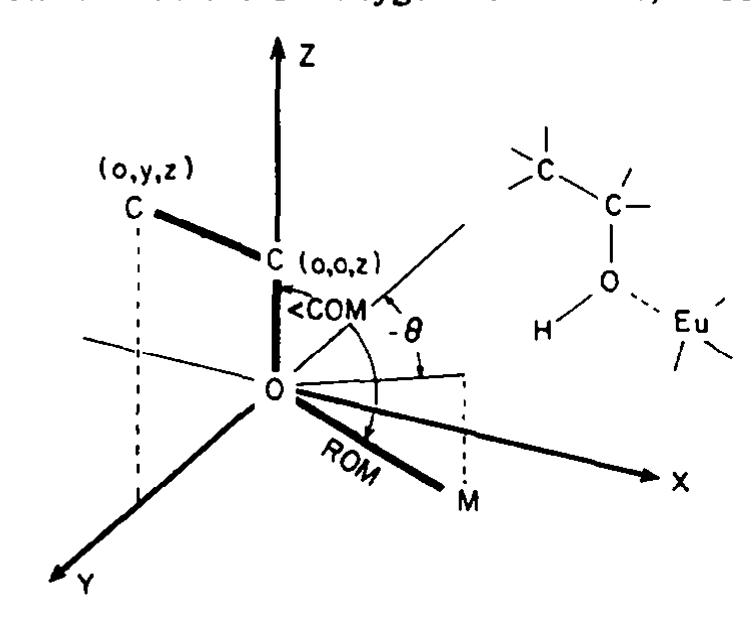










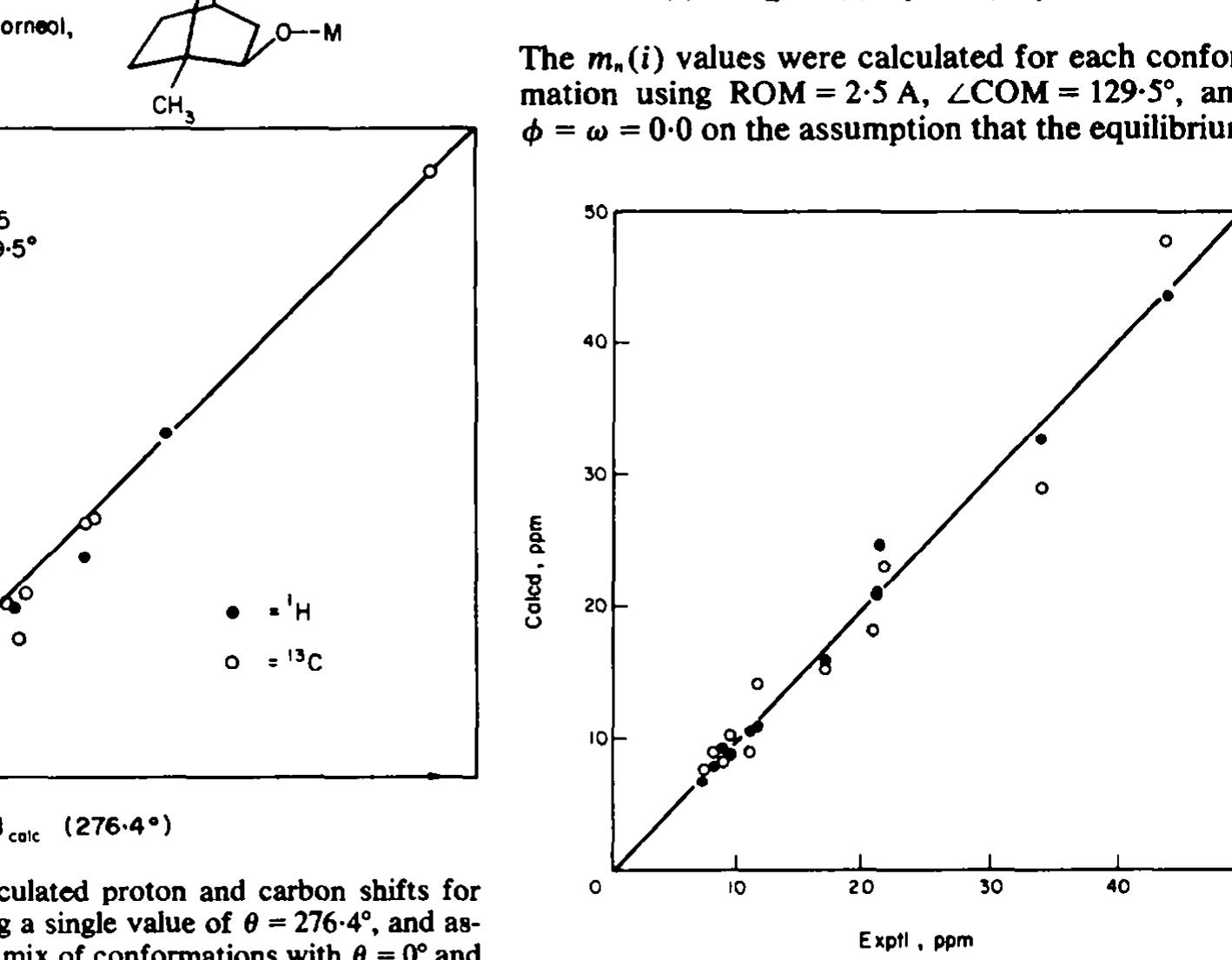
![Fig 11. Plots of calculated shifts of isoborneo! methyl! protons using a single average location of the three protons at a point 0-36 A beyond the methyl carbon along the methyl C—C bond axis (upper line), or the averaged shifts assuming rapid rotation between staggered locations of the methy] protons (lower lines). Other parameters as in Fig 7. A problem arises in conjunction with these same methy] groups as to how the shifts of their hyd- rogens should be calculated. The simple way is to take an average position for all three hydrogens along an extension of the C-C bond, 0-364A beyond the methyl! carbon. Alternatively, one can calculate the individual shifts of the hydrogens for the staggered positions of each Me group vis a vis its point of attachment and assume these are aver- aged by rapid Me rotation. These approaches are not equivalent, especially when the lanthanide atom is close to the Me group. Fig 11 shows the differ- ence between the calculated shifts for the Me hyd- rogens of isoborneol for the two procedures as a function of 6. When @ is 0° or 120°, the lanthanide is rather close to the Me attached to C1 or the syn-Me at C7. For this reason, we have used the individual hydrogen locations for staggered methyl conforma- tions in the LANCON treatment. Annather annrnarh tan datarminatinan af ranfarm.s.](https://figures.academia-assets.com/51447161/figure_014.jpg)






![The sensitivity of the imaging system was compared to silver staining using Cy3 labeled BSA (Fig. 2). The gel was first imaged for fluorescence, and then silver stained [10]. The limit of detection of the fluorescence imager Figure 2. Comparison of fluorescence detection versus silver staining. (A) Serial dilutions of Cy3-labeled BSA were run on a 10% SDS- PAGE minigel and detected using fluorescence imaging. The first two lanes were exposed for 3 min, whereas lanes (3)—(5) were exposed for 15 min, since the first two lanes saturated the detector after 15 min of exposure. (B) Same gel as in (A), imaged after silver staining. (C) Same as (A), except less BSA was used and CCD exposure was lengthened to 30 min. (D) Same gel as in (C), imaged after silver staining. The arrowheads point to the BSA band. The doublet imme- diately underneath the BSA band is a commonly seen contaminant in the sample buffer that appears after “staining to maximum sensitivity” {12}.](https://figures.academia-assets.com/51237055/figure_002.jpg)
![Figure 3. DIGE of BSA-spiked Drosphila embryo extract. (A) Image of Drosphila extract spiked with BSA to a final concentration of 0.1% w/w of total protein and labeled with Cy3. Axis arrows indicate the direction of increasing p/ and M,. The white arrow points to the BSA spots CCD exposure time was 3 min. (B) Image of the unspiked extract, labeled with Cy5. (C) Top: ratio image (Cy3/Cy5) of the BSA region from the gel in (A) and (B). The arrowheads point to individual BSA spots. Bottom: line plot showing the fluorescence intensity from a row of pixels bisecting the horizontal center of the BSA spots in the Cy3 (-@-) and Cy5 (—A--) images. The values were first normalized to equalize total fluorescence intensity. (D)—(F) As in (C), but from ratio images of gels with a range of BSA spikes and CCD exposure times, as follows: (D) BSA, 0.05% w/w; CCD exposure, 10 min; (E) BSA spike, 0.02% w/w; CCD exposure, 25 min; (F) BSA spike, 0.005% w/w; CCD exposure 30 min. The dashed rectangle encompasses the area in which the BSA spike can be detected in the other images; it was not detectable in this experiment. Notice thal since BSA resolves into multiple spots in both (D) and (E), the limit of detection for a single spot is 0.01% w/w teins with fluorescent molecules either prior to FEF [12] or between the [EF and SDS-PAGE steps [13, 14] is not a novel idea. However, previously, only a single fluoro- phore was used for nonspecific protein labeling. Further- more, others have differentially labeled two samples, but using two different radionuclides [13]. The main diffi- teins with fluorescent molecules either prior to IEF [12] or between the IEF and SDS-PAGE steps [13, 14] is not a novel idea. However, previously, only a single fluoro-](https://figures.academia-assets.com/51237055/figure_003.jpg)
![Figure 4. DIGE of induced GAL4VP16 protein. £. coli transformed with the chimeric protein GAL4VP16 [15] were induced for 15 min with IPTG. Protein extract from the induced culture was compared to proteins from uninduced bacteria using analytical DIGE. (A) The induced extract, labeled with Cy3. The large arrowhead indicates the GAL4VP16 protein. A smaller arrowhead indicates another difference, which may be induced B-glactosidase, given its M,. (B) Uninduced extract labeled with CyS. (C) The ratio of Cy3/Cy5. CCD exposure was for 3 min. Axis arrows point in the direction of increasing M, and pl.](https://figures.academia-assets.com/51237055/figure_004.jpg)













![Fig. 1. Summary of published areas for fluid phase DPPC at 50°C (black) and gel phase DPPC (grey) at 20°C. References: Sun et al. [4], ’Pace and Chan [9], °Biildt et al. [10], “Schindler and Seelig [11], “Nagle et al. [3], ‘Lewis and Engelman [12], ®Rand and Parsegian [1] and Janiak et al. [13], "DeYoung and Dill [14], ‘Lis et al. [15], /Thurmond et al. [16]. At this point some readers may challenge our as- sertion that lipid bilayer structure should still be con- sidered an active area. It has a long and rich history. Many prominent biophysicists have published in it and moved on. Users of bilayer structural data have many references to choose from and each user has a favorite. Such a reader should examine Fig. 1 which shows literature values for a particularly cen- ral quantity, namely, the average interfacial area A per lipid molecule for DPPC bilayers at 50°C in the biologically relevant, fully hydrated, fluid (F, synon-](https://figures.academia-assets.com/45249445/figure_001.jpg)
![Fig. 2. Three representations of structure of DPPC bilayers in the Ly fluid phase. (a) Probability distribution functions p for different component groups from simulations [20] and the downward pointing arrows show the peak locations determined by neutron diffrac- tion with 25% water [10]. The equality of the areas denoted o and £ locates the Gibbs dividing surface for the hydrocarbon region de- termined by the simulation. (b) Electron density profile p* from X-ray studies (solid line) [3] and from simulations (dots) (contributed by Scott Feller). (c) Two volumetric pictures. The version on the left monolayer is a simple three compartment representation. The version on the right monolayer is a more realistic representation of the interfacial headgroup region [26]. Dc is the experimentally determined Gibbs dividing surface for the hydrocarbon region. The x-axis is in A along the bilayer normal with the same scale for a, b and c. The y-axis in c shows a lateral dimension along the surface of the bilayer. Values for the parameters in c are taken from Table 6. J.F. Nagle, S. Tristram-Nagle/ Biochimica et Biophysica Acta 1469 (2000) 159-195](https://figures.academia-assets.com/45249445/figure_002.jpg)
![Fig. 3. Schematic view of MLVs with defect regions of excess water. Figure reproduced from [63] with permission of the authors.](https://figures.academia-assets.com/45249445/figure_003.jpg)
![Fig. 4. Snapshot of fluctuations from a non-atomic level Monte Carlo simulation [91].](https://figures.academia-assets.com/45249445/figure_004.jpg)

![Fig. 6. The solid line shows the continuous transform F(q) for fully hydrated gel phase DPPC. The data points show the dis- crete form factors F;, for h= 1-10 for five different values of D from 58.7 A to fully hydrated 63.2 A. The phase factors are in- dicated by the signs under each lobe. The first five phase fac- tors are obvious. The next five require more detailed analysis [39,49].](https://figures.academia-assets.com/45249445/figure_006.jpg)
![Fig. 7. Dependence of A versus ADwP for DOPC at 30°C [38]. The solid line is the best fit with slope —1/K, corresponding to Ka = 188 dyn/cm. The dotted line is the best fit using Ka = 265 dyn/cm from [17].](https://figures.academia-assets.com/45249445/figure_007.jpg)
![Fig. 8. Osmotic pressure P versus steric water space Dw’. Vari- ous lines show contributions from various interactions, with the bold solid curve showing the fitted total P. It has been proposed for the soft confinement re- gime that the fluctuation interaction free energy in Eq. 11 should be modified [86,87] and a formula where H is the Hamaker parameter. This is the in- teraction assumed to be responsible for limiting the swelling in bilayers composed of lipids with no net charge. We define Dwo’ to be the limiting water space for fully hydrated MLVs with osmotic pressure P=0. Because Dwo’ is only 10-30 A,a graph of bare potential Vg versus Dw’ on this length scale shows considerable variation. For charged lipids in low salt, one should also consider an electrostatic interaction, but this is absent for the neutral lipids. An additional very short-range repulsion has been measured and attributed to headgroup protrusions [90]. We do not include it since it only plays a role for lipids under high osmotic pressure and small water space Dw’. It does, however, play the formal role of sup- pressing the singularity in the van der Waals poten- tial at Dw’ =0.](https://figures.academia-assets.com/45249445/figure_008.jpg)


![Fig. 11. Electron density map obtained using X-ray phases from [147] and intensity data from [146] for the ripple thermo- dynamic phase of DMPC with 25% water (mw =13) at 18°C. The rippling repeat period is 142 A (length of unit cell) and the lamellar repeat is 58 A (height of unit cell). The profiles show a major M side (across A) that has the same thickness as the gel phase and a thinner minor m side (across B). The presence of a thin water layer between bilayers (across C) indicates complete inner shell hydration of the headgroups. Most lipids have more low temperature chain or- dered phases than just the gel phase. The most strik- ing of these is the rinnle phase. which occurs just](https://figures.academia-assets.com/45249445/figure_011.jpg)

![*This work. bArmen et al. [22]. Volumes of component groups for general Ly phase lecithins ignoring temperature dependence](https://figures.academia-assets.com/45249445/table_002.jpg)
![All areas in A2; RT, room temperature. For adjusted comparisons, see Table 5. References: 'Lis et al. [15], ?Janiak et al. [13], 3Gruner et al. [61], *Reiss-Husson [60], “Small [62], “Tardieu et al. [42], 7McIntosh et al. [64], $Rand and Parsegian [1], Koenig et al. [63], '°Sun et al. [4], ''Nagle et al. [3], '?Petrache et al. [36], !*Tristram-Nagle et al. [38], 'Biildt et al. [10], "Wiener and White [25], '6Lewis and Engelman [12]. Comparison of literature values for area/lipid* Table 3](https://figures.academia-assets.com/45249445/table_003.jpg)
![Data used to make corrections “Temperature adjusted to 30°C. >Estimated, but see new results in text from Evans (private communication). “All osmotic pressures are in atmospheres (10° dyn/cm’). ‘Estimated from [70]. Table 4](https://figures.academia-assets.com/45249445/table_004.jpg)
![Adjusted areas (A2) for results in Table 3 The original results given in the corresponding location in Ta- ble 3 have been adjusted due to “Temperature (see Section 7) ‘Fluctuation geometry (see Section 7.1) °New Ka [17] ‘Dc (see Section 8.1) *Patterson truncation and Dy; reduction (see Section 10.1). Agx = A[Dp + (Dw)]/D = A(cos@)](https://figures.academia-assets.com/45249445/table_005.jpg)