Primary minerals of the Jachymov ore district
2003, Journal of Geosciences

Sign up for access to the world's latest research
Abstract
One hundred and seventeen primary mineral species are described and/or referenced. Approximately seventy primary minerals were known from the district before the present study. All known reliable data on the individual minerals from Jáchymov are presented. New and more complete X-ray powder diffraction data for argentopyrite, sternbergite, and an unusual (Co,Fe)-rammelsbergite are presented. The following chapters describe some unknown minerals, erroneously quoted minerals and imperfectly identified minerals. The present work increases the number of all identified, described and/or referenced minerals in the Jáchymov ore district to 384.
















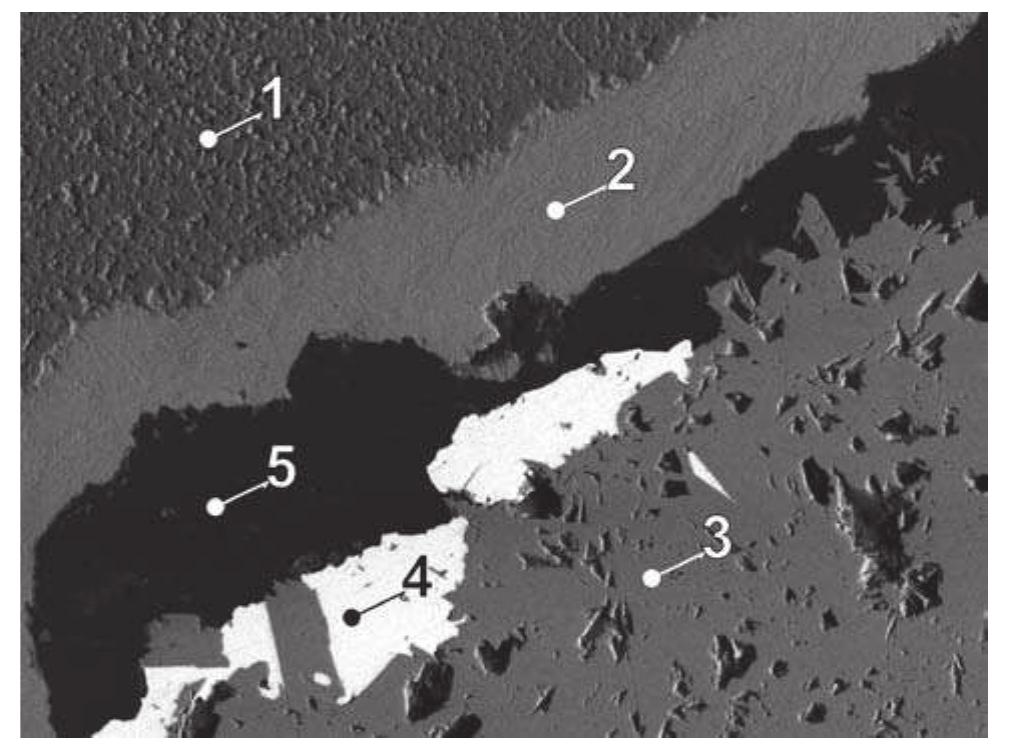
![Fig. 5. JL16P/D-2. 1 — annabergite, 2 — pyrite, 3 — Ag-Fe-sulphides. Svornost shaft, 8 level, Geschieber vein. BSE image. Magnification 270x. Argentopyrite was decribed from Jachymov by Sarto- rius in 1866 [438]. Tschermak [469] considered the](https://figures.academia-assets.com/44383943/figure_005.jpg)







![Fig. 13. Argentopyrite according to Schrauf in [492].](https://figures.academia-assets.com/44383943/figure_013.jpg)

![Fig. 14. Comparison of distorted six-member rings S-Fe-S—Fe—S—Ag in the structure of argentopyrite (top) and sternbergite [430] (bottom). Two Fe atoms are positioned above the triangle defined by S atoms and Ag atom is on the opposite side of the triangle.](https://figures.academia-assets.com/44383943/figure_014.jpg)





















![Fig. 27. Composition of arsenpolybasite from Jachymov compared with the solid solution in a synthetic system [441].](https://figures.academia-assets.com/44383943/figure_027.jpg)





























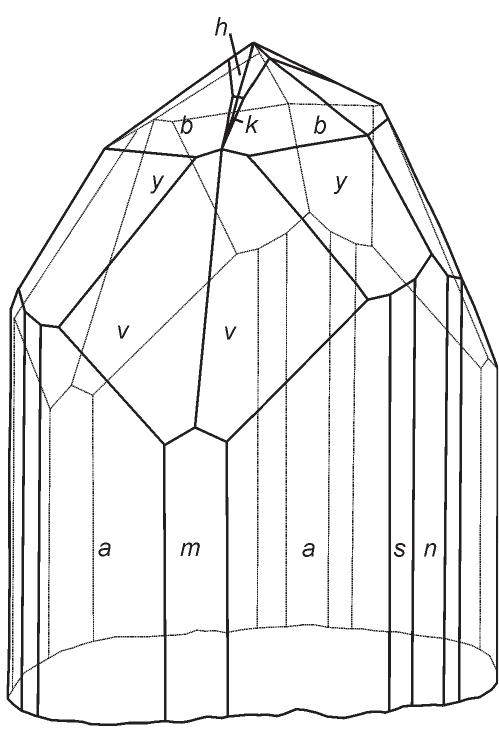
![Fig. 50. Chalcocite crystal drawing by Vrba [362]. Crystal forms: c(001), e(012), d(021), b(010), z(113), v(112), p(111), m(110), n(230), m(130), a(100).](https://figures.academia-assets.com/44383943/figure_049.jpg)















![* The value estimated from unit-cell dimensions and thermal analysis. Theoretical value for USiOs is 7.16 g.cm™® [150]. Table 39. Calculated unit-cell parameters of coffinite from Jachy- mov for the space group /4,/amd and density.](https://figures.academia-assets.com/44383943/table_042.jpg)









![Table 43. Microhardness of diaphorite. Diaphorite forms anhedral to subhedral grains of micro- scopic size in microcrystalline intergrowth with pyrite and stephanite. These coatings cover massive stephan- ite covered by stephanite crystals. The specimen collect- ed in 1872 is in the Mineralogical collection, National Museum, Prague, No. NM9512 [333].](https://figures.academia-assets.com/44383943/table_047.jpg)



![Fig. 62. Dickite crystals. SE image. Magnification 690x. Phot¢ Z. Mach [595].](https://figures.academia-assets.com/44383943/figure_063.jpg)














![Table 57. Chemical analyses of freibergite. white) but fluorite of the carbonate-uraninite stage is violet turning to nearly black in proximity of uraninite lenses. In both cases, fluorite forms isolated grains, small aggregates or thin veinlets with euhedral crystals in vugs [351]. Galena PbS](https://figures.academia-assets.com/44383943/table_060.jpg)






















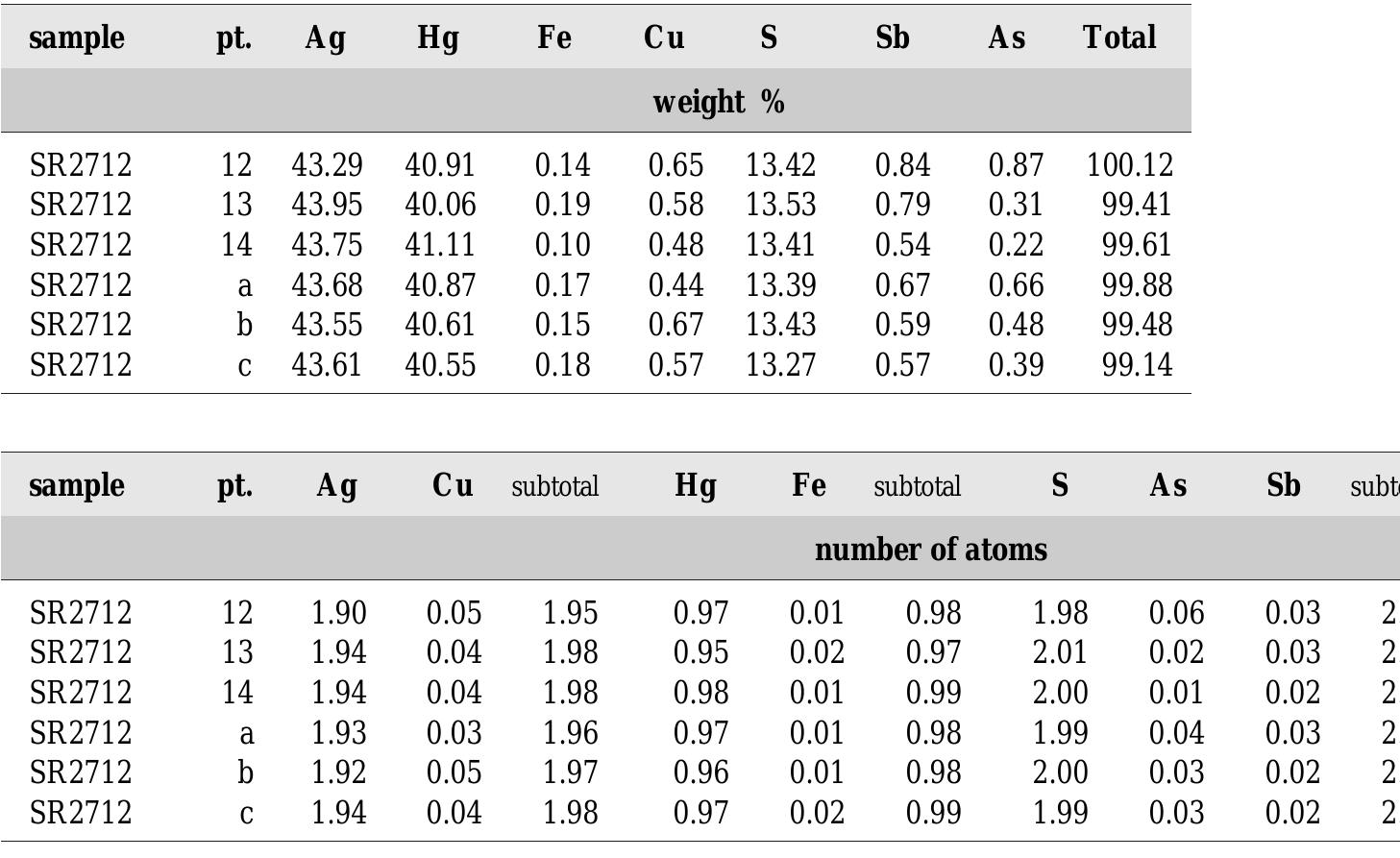












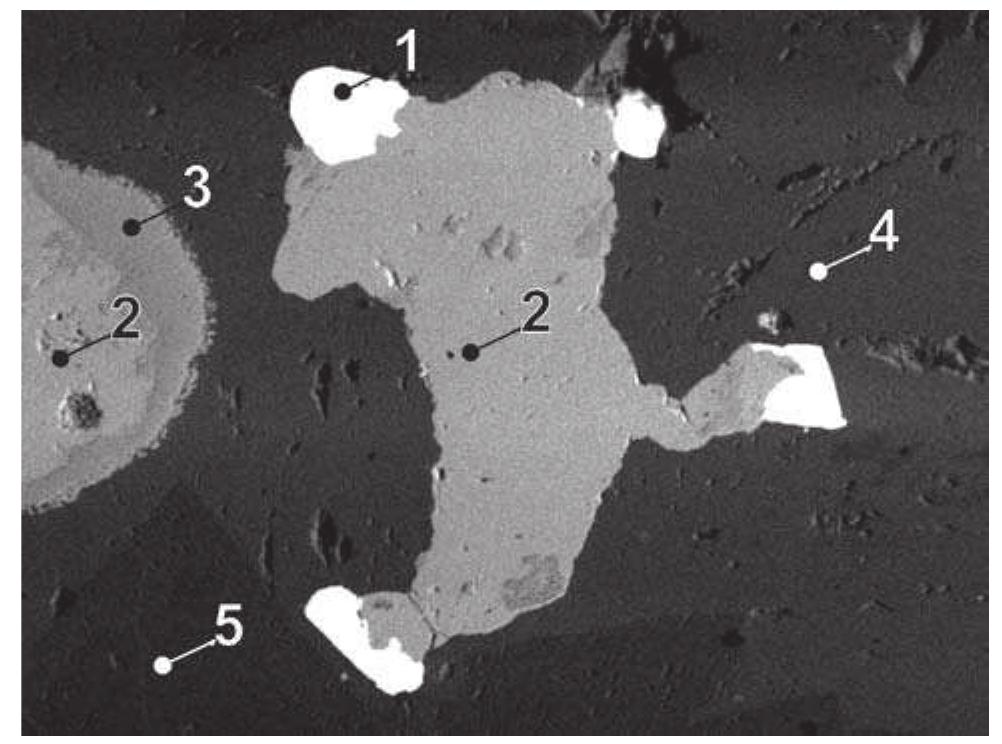
![Fig. 89. JO74P/A-3. 1 — lautite, 2 — ldllingite, 3 — bornite, 4 — galena, 5 — quartz. Svornost shaft, Daniel level, intersection of Trojicka vein with Geschieber vein. BSE image. Magnification 320x. A possibility remains that enargite replacing bismuth, reported by Ziikert [423] in a polished section, was also lautite, because of similarity in optical properties of enargite and lautite.](https://figures.academia-assets.com/44383943/figure_091.jpg)














































![Table 89. Calculated unit-cell parameters of nickel- skutterudite from Jachymov for the space group /m3. The second type of nickel-skutterudite — cubic crystals or rather their relics partly replaced by bismuth and quartz, and some anhedral crystals, associate not only with bismuth and bismuthinite but also with tennantite, chalcopyrite and younger sulphides. Density corrected for content of bismuth and quartz was 6.23 g/cm? [429]. According to Zepharov- ich [391], density of nickel-skutterudite is 6.89 g/cm’.](https://figures.academia-assets.com/44383943/table_095.jpg)


























![Table 97. Calculated unit-cell parameters of proustite from Jachymov for the space group R3c. Proustite-pyragyrite minerals close in composition to the end-members occur only exceptionally in the Jachymov ore veins. Pyrargyrite has at least a minor As content, with minimum at 0.2 wt.% As. Both minerals tend to form in- tergrowth in crystallographic orientation and single crys- tals of pure proustite and pyrarargyrite have not been found. Ramdohr [487] described crystallographically ori- entated intergrowth of the two minerals from Andreasberg in Harz, Germany. From Jachymov, Ramdohr mentioned](https://figures.academia-assets.com/44383943/table_105.jpg)

















![Fig. 147. Correlation of unit-cell parameter a and As content for pyrites enriched in As from Jachymov. Red symbols show data for As-free¢ pyrite, taken from [499].](https://figures.academia-assets.com/44383943/figure_150.jpg)




















![Table 106. (continued) The outer shape of rammelsbergite aggregates is usu- ally irregular or botryoidal and euhedral crystals are rare. Rammelsbergite rims around native metals show a zon- ing structure (Fig. 150), with individual zones showing different resistence to oxidation in air. The internal struc- ture of zoned aggregates indicates that the zones mimic shape of dendritic silver, serving as matrix for rammels- bergite. It sometimes forms radiating aggregates [351]. The present study confirmed observations by Mrna and Pavlt [351] and characterizes rammelsbergite as belong- ing to abundant minerals in the deposit. It is newly found in rims around bismuth. It also forms individual grains or larger aggregates. It shows a strong anisotropy and ag- gregate extinction in polished sections. It is often asso- ciated with nickel-skutterudite. Reported zoning of ram- melsbergite aggregates may be explained by variation in](https://figures.academia-assets.com/44383943/table_117.jpg)











![Table 113. Calculated unit-cell parameters of W-Sn rutile from Jachymov for the space group P4,/mnm. ization stage, contain rutile up to 100 um long, showing sharply defined growth zones in BSE images. Light zones correspond to rutile with 4-6 wt.% W, 2-2.5 wt.% Fe, 1-1.5 % Sn and dark zones have 0.2-0.7 wt.% W, 0.9-2.4 wt.% Fe, and 0.8-1.4 % Sn. Rocks containing this zoned W-rich rutile carry topaz. A similar W-rich rutile was described from Western Australia [387] both from muscovite schist and quartz vein. It contains 7 wt.% WO,, 2 wt.% Sb,O, and 1 wt.% FeO.](https://figures.academia-assets.com/44383943/table_124.jpg)




























































![Fig. 200. Drawing of sternbergite crystal and twin by Haidinger [492].](https://figures.academia-assets.com/44383943/figure_205.jpg)





















![Vladimir shaft, M-2 vein, contains several tetrahedrite grains enclosed in bornite veinlet. Other well-formed tet- rahedrite crystals come from collection of National Mu- seum, Prague (Fig. 213). neous. This is shown by variation in chemical analyses, particularly in Pb, Zr and Y contents. The increased Si con- tents, interpreted in [150] by entry of Si into interlayer po- sitions along [111], could be alternatively interpreted by a heterogeneous admixture of amorphous gels SiO, - nH,O. The latter interpretation would correspond to geochemi- Tetrahedrite is rare in Jachymov though local tennan- tites contain a common admixture of Sb.](https://figures.academia-assets.com/44383943/table_159.jpg)

![Fig. 213. Well-formed tetrahedrite crystals on calcite (width of figure 1.1 cm). Photo J. & E. Sejkora. Table 143. Calculated unit-cell parameters of tetrahedrite from Jachymov for the space group [43m [443].](https://figures.academia-assets.com/44383943/figure_218.jpg)



















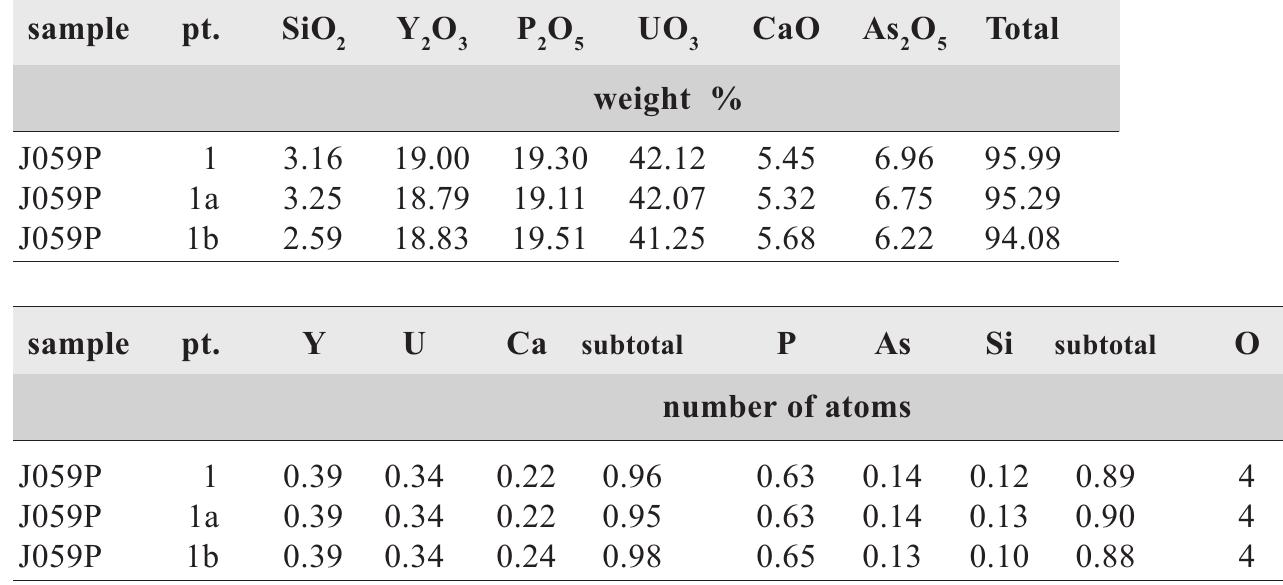









![Fig. 228. VS7651. Crystals of “frieseite”. XRD analysis confirmed that the mineral is a mixture of sternbergite, pyrite and marcasite. Magnification 30x. Photo A. GabaSova. Vrba [532] studied several samples from the Hildebrand and Geister veins with sternbergite and argentopyrite. Two samples representing the two veins contained a min- eral similar to sternbergite but showing different crystal morphology. Vrba introduced the name “frieseite”’ for this](https://figures.academia-assets.com/44383943/figure_233.jpg)





![Fig. 234. Thick tabular singlecrystal of “frieseite” from Jachymov and a twin by Vrba [362]. Crystal forms: c(001), b(010), w(301), r(102).](https://figures.academia-assets.com/44383943/figure_239.jpg)
![Fig. 235. Orientated intergrowth of a thick tabular crystal of “frieseite” and argentopyrite, by Vrba [362]. Crystal forms: c(001), b(010), q(043), (102), y(101), w(301), t(131).](https://figures.academia-assets.com/44383943/figure_240.jpg)
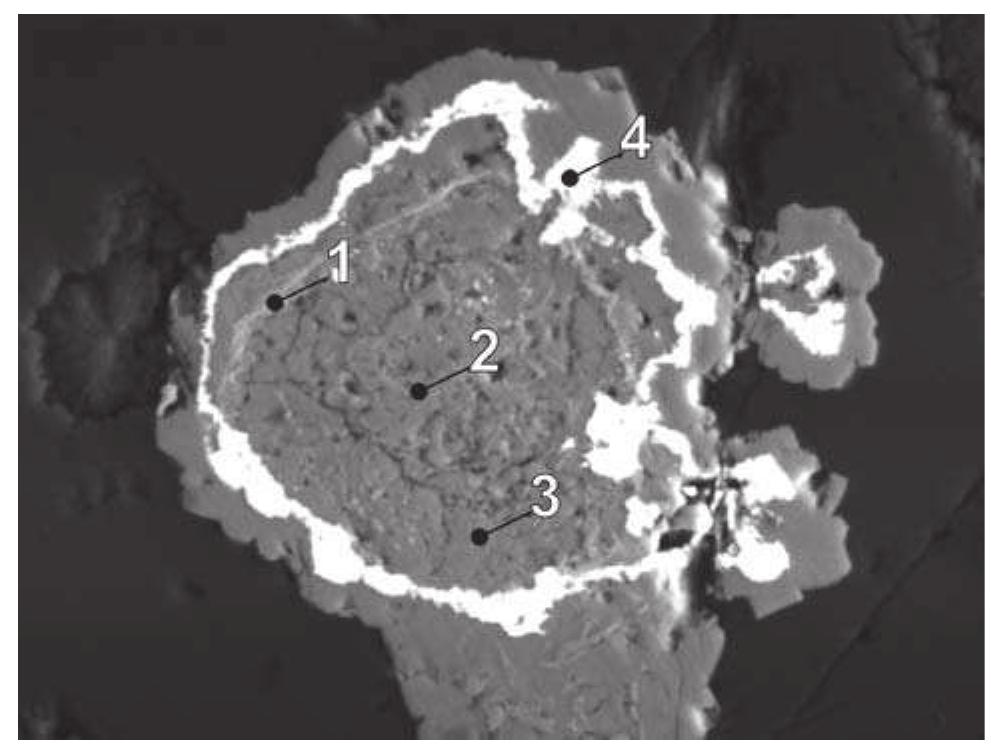
![Fig. 237. Interpretation of Mrna and Pavlu [383] — maucherite rims a fissure in nickeline, arsenide mineralization stage. Eva shaft, vein No. 39. Magnification 80x. Fig. 236. Topotactic replacement of nickeline along small fissures by orientated intergrowths of rammelsbergite (NAP), from [354]. 1 — NAP, 2 — nickeline, 3 — Ni-arsenete? Germany. Reflected light, single polarizer. Magnification 120x.](https://figures.academia-assets.com/44383943/figure_241.jpg)
![No maucherite was identified during the present study, including some original samples. Alteration of nickeline may possibly result in formation of a rare phase NAP (nickeline alteration product), described by Karup-M@ller and Makovicky [354] in an old sample from an unspeci- fied locality in Germany. The phase NAP evolves by par- tial alteration of nickeline along fractures as lamellae 2x20 um in parallel orientation. Border between NAP](https://figures.academia-assets.com/44383943/figure_242.jpg)
Related papers
Journal of Geosciences, 1997
Two hundred and seven secondary mineral species are described and/or referenced. Approximately seventy secondary minerals were known from the district before the present study. All known reliable data on the individual secondary minerals from Jáchymov are presented. New and more complete X-ray powder diffraction data for compreignacite, irhtemite, lavendulan, lindackerite, masuyite, mcnearite, metaschoepite, mottramite, rabbittite, rabejacite, richetite, sodium-zippeite, voglite, zellerite, zippeite, and zýkaite are presented. A list of secondary minerals arranged according to chemical composition and list of "non-secondary" minerals are included at the end of this paper.
Journal of Geosciences, 2003
Jáchymov is the type locality for the following primary minerals: argentopyrite, krutovite, millerite, sternbergite, and uraninite. The history of their discovery is described in this contribution. The paper sums up the history of discovery of other type minerals at Jáchymov [560].
Journal of the Czech …, 1997
Jáchymov is type locality for 22 minerals, including 17 secondary minerals. Data on history of discovery and description of new minerals was extracted by search in old literature. Minerals are arranged in the chronological sequence of discovery. Explanation of names of discredited or redefined minerals and some historical names is included at the end of this paper.
Journal of Geosciences, 1997
This paper presents information on relations between physical-chemical properties and genetic features for two hundred and seven secondary minerals and thirty natural phases newly discovered in the Jáchymov ore district. Eight distinct paragenetic groups are described and discussed with respect to formation conditions of the secondary minerals in the Jáchymov ore district.
2003
The paper presents biographic data of persons, after whom new primary minerals discovered at Jáchymov were named (K. Sternberg, W. H. Miller, G. A. Krutov), as well as the recently discovered secondary minerals (J. Vajdák, J. Èejka, J. venek). Biographies of scientists who described new minerals from Jáchymov are given in the following part (F. E. Brückmann, I. Born, A. G. Werner, H. Dauber, G. A. Kenngott, W. Sartorius v. Waltershausen, F. Sandberger, R. Nováèek, R. A. Vinogradova). Biographies of persons who significantly contributed to mineralogy of the Jáchymov ore district are presented in the last section (F. Babánek, J. tìp, R. Zückert, R. P. Dubinkina, R. V. Geceva, F. Mròa, M. Komárek, D. Pavlù). This contribution is a continuation of the 1997 article Who was who? In names of secondary minerals discovered in Jáchymov [561] dealing exclusively with secondary minerals.
Journal of the Czech …, 2003
Ore-forming processes and mineral parageneses of the Jáchymov ore district Rudotvorné procesy a minerální pragenze jáchymovského rudního okrsku
Journal of Geosciences, 1997
Introduction Torbern Olof Bergman was born in Katrineberg, Sweden, the son of Barthold Bergman, sheriff on the royal estate at Katrineberg. Bergman studied mathematics, philosophy, physics and astronomy at the University of Uppsala, graduating in 1756. He later joined the faculty of the University, teaching physics and mathematics, and succeeded to Wallerius chair as Professor of chemistry in 1767. He developed a growing interest in chemistry, mineralogy and crystallography when demonstrated how the stacking of rhombohedral units could produce a scalenohedron. (Haüy later proposed the same thing but denied having known on Bergman's theories.) [217]. Jáchymov is type locality for 17 secondary minerals. Ten of these minerals were named after persons, including some prominent mineralogists of the 19th century. Interesting biographical data on these personalities were assembled during work on the projects of study of the secondary minerals in the Jáchymov ore district (Grant Agency ...
Journal of Geosciences, 1997
This paper describes thirty inorganic compound-secondary mineral phases-found in the nature for the first time. All compounds come from the Jáchymov ore district. All up-to-now available physical and chemical data and references to appropriate literature are given. Crystal structure of phase [ (MoO 2) 2 As 2 O 5 (H 2 O) 2 ]. H 2 O was solved and refined, crystal structures of Ca(H 2 AsO 4) 2 and Mg-villyaellenite were refined by the Rietveld method.
Journal of Geosciences, 2003
Geology and hydrothermal vein system of the Jáchymov (Joachimsthal) ore district Geologie a hydrotermální ilný systém jáchymovského rudního okrsku (26 figs, 1 tab) This contribution provides a brief review of geology of the Kruné hory Mts. (Erzgebirge) and specifically of the Jáchymov (Joachimsthal) deposit. Main types of rocks in the Jáchymov ore district and the role of tectonic processes are described. The position of the ore district is defined much like its tectonic borders. Geological factors controlling the mineralization (especially the so-called five-element mineralization) are summarized. Hydrothermal veins are divided into Morning and Midnight veins according to the classical historical speak of miners, and all vein clusters are listed. Vein hydrothermal five-element mineralization is chronologically divided into separate mineralization stages and their age is estimated.
Geology of Ore Deposits, 2006
Russian Geology and Geophysics, 2019
Ore mineralogy of the Kedrovskoe-Irokinda ore field (northern Transbaikalia) has been studied. The ore field comprises ca. 200 quartz veins. Vein 3 and the Kvartsevaya and Serebryakovskaya veins of the Irokinda deposit and the Shamanovskaya, Pineginskaya, Osinovaya, and Barguzinskaya veins of the Kedrovskoe deposit have been described. Quartz-pyrite assemblage (quartz-1, pyrite, pyrrho-tite, and marcasite) and quartz-gold-sulfide assemblage (quartz-2, galena, chalcopyrite, sphalerite, electrum, fahlore, Ag tellurides, and sulfosalts of Ag, Cu, Sb, Pb, and Sn) have been revealed. Major ore minerals were investigated by EPMA and LA-ICP-MS. An increase in Ag content in electrum (from 5.5 to 72.4 wt.%) and fahlores (from 5 to 35 wt.%) and in the abundance of Ag minerals during the ore formation has been established. Galena contains impurities of Sb and Ag (thousands of ppm), Se, Cd, Te, and Bi (hundreds of ppm), Cu, Zn, As, and Sn (tens of ppm). It is shown that the Kedrovskoe-Irokinda ore field is a rare type of orogenic deposits with considerable variations in the composition of major ore minerals (electrum, sphalerite, and fahlores), which is explained by the diversity of the host rocks.
2015
Metakirchheimerite was found only on a few samples from the Jan Evangelista vein at the “Adit level ” of the Svornost
Journal of Geosciences, 1997
Jáchymov was founded in 1516 in place of the former abandoned village Konradsgrün, following discovery of a rich silver deposit. During the first twenty years, the town witnessed a remarkable boom, becoming in 1534 the second largest town in Bohemia, second only to Prague. Jáchymov became an important cultural centre, hosting Agricola, Mathesius and many outstanding personalities of the time. Latin school was founded in Jáchymov; a major part of library assembled in this school was preserved till present. The coins minted in Jáchymov were named after the German name of the town "Thaler", later "tolar", the name which during time was adopted as the name of the USA currency. A mining school was founded in Jáchymov in 1716. The history of the town witnessed periods of expansion and episodes of decline due to wars, fires, plague epidemic waves, and economic problems. The original interest in silver was substituted by mining of cobalt and nickel, later on, uranium was used for production of uranium colours and separation of radium compounds for medical application. The last major mining expansion took place after the Second World War, with extensive mining and ore dressing of uraninite and its exports to USSR. The ore district was exhausted and mining terminated by 1964. Springs of radioactive water taped in the mines are used since 1906 for curative application in local spa.
A rare , unique , Gem Rubellite Tourmaline from Pala mine in California , USA .
Review of the Bulgarian Geological Society, 2020
American Mineralogist, 2016
Two new uranyl sulfates, geschieberite (IMA 2014-006), ideally K 2 (UO 2)(SO 4) 2 (H 2 O) 2 and svornostite (IMA 2014-078), ideally K 2 Mg[(UO 2)(SO 4) 2 ] 2 •(H 2 O) 8 were recently discovered in the Geschieber vein at the Svornost mine, Jáchymov (Joachimsthal), Western Bohemia, Czech Republic, and named for their type locality. The Jáchymov ore district is a classic example of the Variscan hydrothermal vein type of deposit, so-called five-element formation, Ag-Bi-Co-Ni-U. Both new minerals are the supergene products of the post mining alteration of the uraninite and sulfides of the primary ore. They occur in the close association with each another and with adolfpateraite, gypsum and mathesiusite. Both minerals exhibit strong yellowish green fluorescence under both short-and long-wave UV radiation. Both are brittle, have an uneven fracture, and estimated Mohs hardness of ~2. Both are unstable under electron beam so the EDS mode was chosen for electron probe measurements. Geschieberite forms bright green, compact crystalline aggregates composed of multiple intergrowths of prismatic {010} crystals elongated on [001] typically 0.1-0.2 mm across sometimes modified by {001}. The crystals are translucent pale green with greenish-white streak and a vitreous luster. The cleavage is perfect on {100}. The density could not be measured due to paucity of pure material; D calc = 3.259 g/cm 3. Geschieberite is slightly soluble in cold H 2 O. It is optically biaxial (-), β = 1.596(2), γ = 1.634(4) (590 nm); X = a. In a plane-polarized transmitted light, the mineral is nearly colorless with no apparent pleochroism. The average of 7 electron probe EDS analyses is [wt% (range)]: Na 2 O 0.23 (0.12-0.59), K 2 O 14.29 (12.90-16.66), MgO 2.05 (1.77-2.52), CaO 0.06 (0-0.12), UO 3 49.51 (47.23-51.64), SO 3 27.74 (26.82-28.78), H 2 O 6.36 (by structure refinement), total 100.24. This gives the empirical formula (K 1.72 Mg 0.29 Na 0.04 Ca 0.01) Σ2.06 (U 0.98 O 2)(S 0.98 O 4) 2 (H 2 O) 2 based on 12 O apfu. The strongest lines in the X-ray powder-diffraction pattern [d Å (I%; hkl)]
Journal of GEOsciences, 2012
This paper presents results of study of supergene minerals occuring at the Huber stock and Schnöd stock in the Krásno Sn-W ore district near Horní Slavkov (Slavkovský les area, Czech Republic). The mineralogical research is based on X-ray powder diffraction, electron microprobe analyses, optical and electron microscopy. The paper includes encyclopaedia-type presentation of the identified mineral species. The role of late hydrothermal, supergene, sub-recent and recent processes in the formation of minerals and their associations is discussed.
Mineralogical Magazine, 2012
Electron-microprobe analyses of Russian and Mongolian chevkinite-group minerals from little-known host lithologies, including various metasomatic rocks, quartzolites and an apatite deposit, are presented. The mineral species analysed include chevkinite-(Ce), perrierite-(Ce), polyakovite-(Ce) and Sr-and Zr-rich perrierite-(Ce). Compositional variation in the Sr-rich members of the group is broadly represented by the exchange vector (Fe + Mn + Al + REE) $ (Ca + Sr + Ti + Zr). Despite the varied parageneses, the chevkinite-(Ce) compositions are similar to previously published data. Many crystals have strong internal compositional variations, partly produced during primary crystallization and partly during low-temperature hydrothermal alteration.
The Canadian Mineralogist, 2016
Grains of the bowieite-kashinite solid-solution series were characterized by electron microprobe analysis, single-crystal Xray diffraction, and Raman spectroscopy. The grains were recovered from dunitic rocks located in the Svetly Bor Ural-Alaskan type massif, Urals, Russia. Bowieite and kashinite occur as small inclusions (up to 100 lm in size) in isoferroplatinum grains up to 1 mm in diameter. Single-crystal X-ray diffraction data for two selected grains, with compositions (Rh 1.16 Ir 0.82 Cu 0.02 ) R2.00 S 3.00 and (Ir 1.06 Rh 0.87 Cu 0.04 ) R1.97 S 3.03 , confirm that the minerals are orthorhombic with similar cell dimensions: a 8.46(1), b 6.00(1), c 6.14(1)Å for bowieite, and a 8.46(1), b 5.99(1), c 6.14(1)Å for kashinite. The Raman spectra of the same two single grains show similar characteristic bands. One grain with composition (Ir 0.26 Rh 0.13 Pt 0.12 Ni 0.18 Cu 0.18 Fe 0.11 ) R0.98 S was found associated with the kashinite. It shows a distinctive Raman spectrum and may represent a new platinum-group mineral, although the small size (less than 20 lm) prevented us from obtaining X-ray diffraction data. Our data for the bowieite-kashinite series are compared with selected worldwide occurrences.
Related topics

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
 Vladimir Srein
Vladimir Srein
