Primary minerals of the Jachymov ore district
2003, Journal of Geosciences
…
129 pages
1 file

Sign up for access to the world's latest research
Abstract
One hundred and seventeen primary mineral species are described and/or referenced. Approximately seventy primary minerals were known from the district before the present study. All known reliable data on the individual minerals from Jáchymov are presented. New and more complete X-ray powder diffraction data for argentopyrite, sternbergite, and an unusual (Co,Fe)-rammelsbergite are presented. The following chapters describe some unknown minerals, erroneously quoted minerals and imperfectly identified minerals. The present work increases the number of all identified, described and/or referenced minerals in the Jáchymov ore district to 384.
Figures (418)













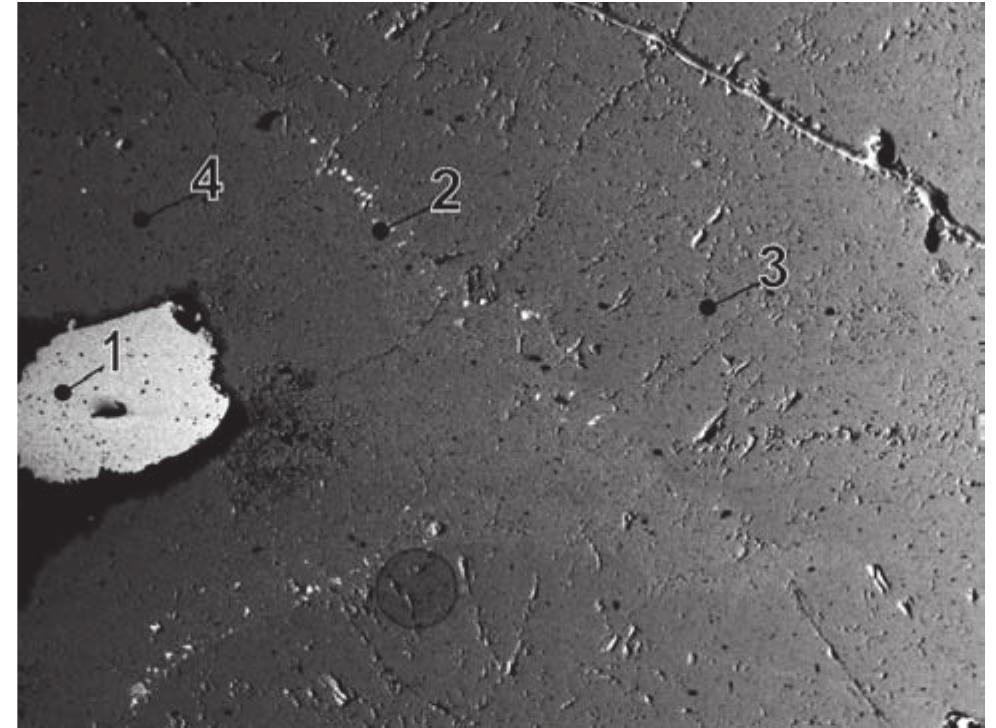
![Fig. 5. JL16P/D-2. 1 — annabergite, 2 — pyrite, 3 — Ag-Fe-sulphides. Svornost shaft, 8 level, Geschieber vein. BSE image. Magnification 270x. Argentopyrite was decribed from Jachymov by Sarto- rius in 1866 [438]. Tschermak [469] considered the](https://figures.academia-assets.com/71852809/figure_005.jpg)







![Fig. 13. Argentopyrite according to Schrauf in [492].](https://figures.academia-assets.com/71852809/figure_013.jpg)

![Fig. 14. Comparison of distorted six-member rings S-Fe-S—Fe—S—Ag in the structure of argentopyrite (top) and sternbergite [430] (bottom). Two Fe atoms are positioned above the triangle defined by S atoms and Ag atom is on the opposite side of the triangle.](https://figures.academia-assets.com/71852809/figure_014.jpg)






















![Fig. 27. Composition of arsenpolybasite from Jachymov compared with the solid solution in a synthetic system [441].](https://figures.academia-assets.com/71852809/figure_028.jpg)








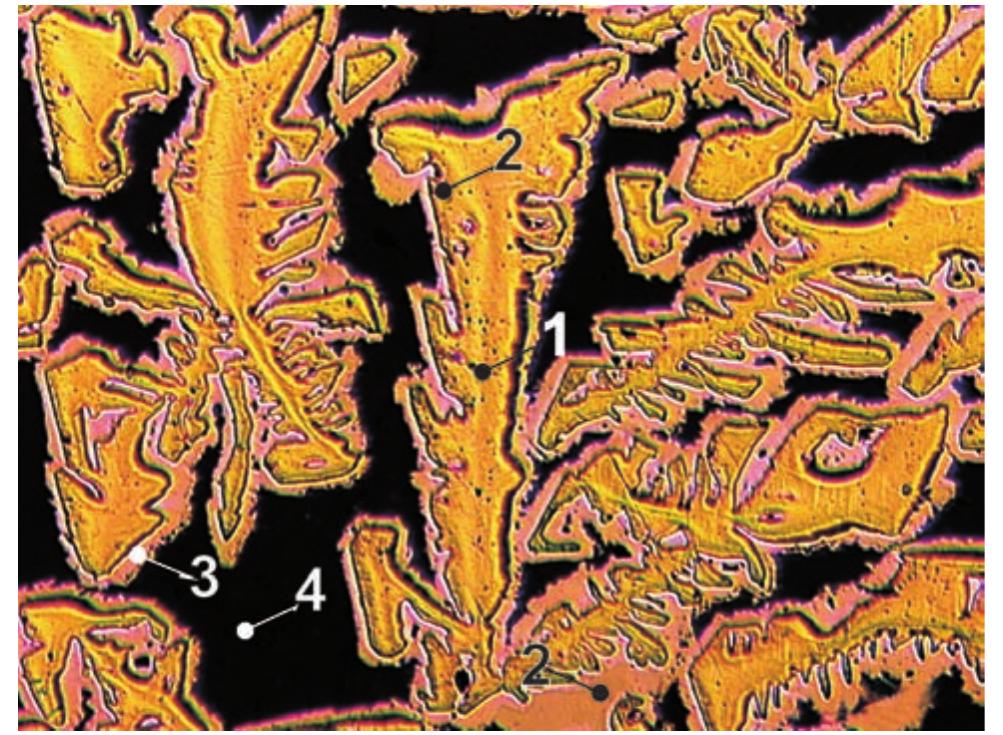


















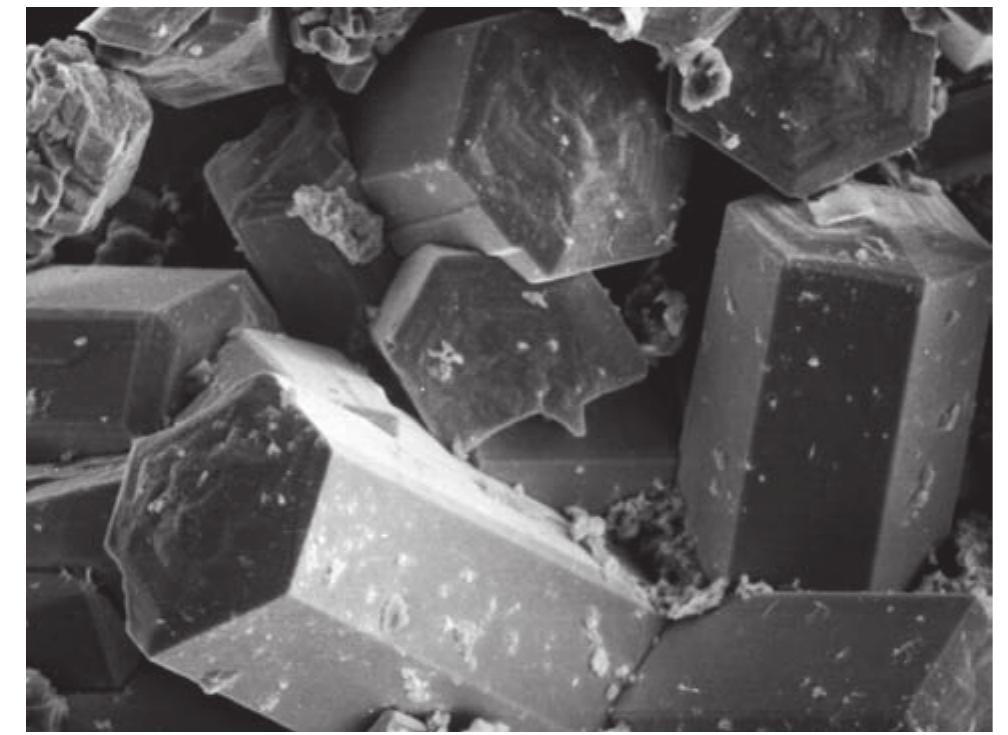
![Fig. 50. Chalcocite crystal drawing by Vrba [362]. Crystal forms: c(001), e(012), d(021), b(010), z(113), v(112), p(111), m(110), n(230), m(130), a(100). Fig. 49. Group of tabular crystals of chalcocite up to 2 mm (width of figure 5 mm). Photo J. & E. Sejkora.](https://figures.academia-assets.com/71852809/figure_049.jpg)

















![* The value estimated from unit-cell dimensions and thermal analysis. Theoretical value for USiOs, is 7.16 g.cm” [150]. Table 39. Calculated unit-cell parameters of coffinite from Jachy- mov for the space group /4,/amd and density.](https://figures.academia-assets.com/71852809/table_041.jpg)









![Table 43. Microhardness of diaphorite. Diaphorite forms anhedral to subhedral grains of micro- scopic size in microcrystalline intergrowth with pyrite and stephanite. These coatings cover massive stephan- ite covered by stephanite crystals. The specimen collect- ed in 1872 is in the Mineralogical collection, National Museum, Prague, No. NM9512 [333].](https://figures.academia-assets.com/71852809/table_046.jpg)



![Fig. 62. Dickite crystals. SE image. Magnification 690x. Photc Z. Mach [595].](https://figures.academia-assets.com/71852809/figure_064.jpg)











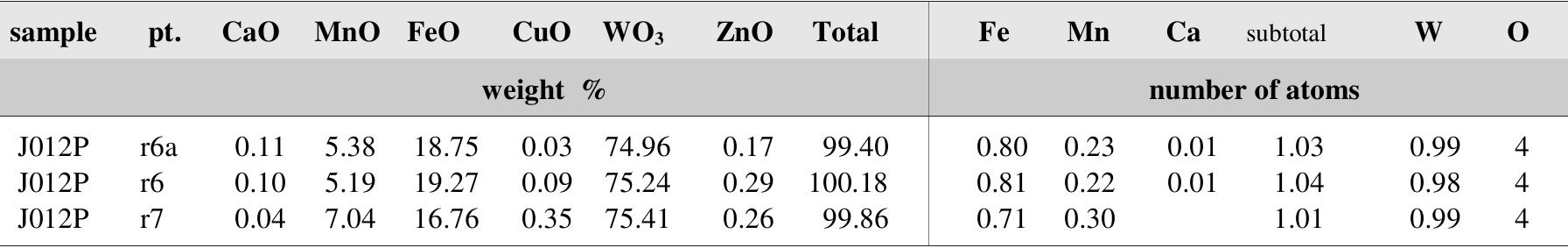



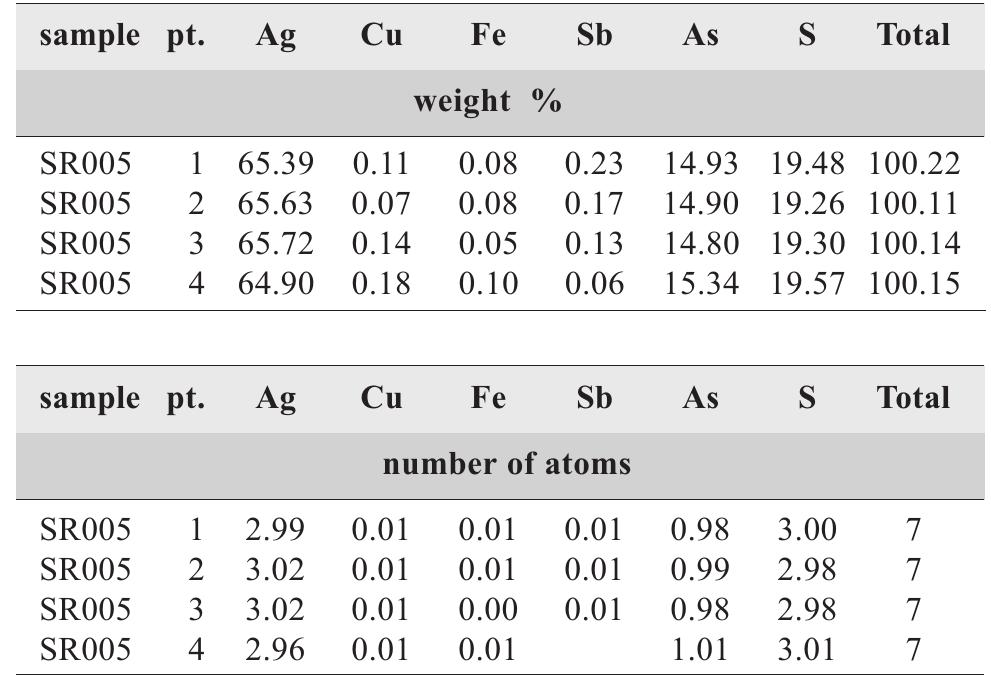
![Table 57. Chemical analyses of freibergite. white) but fluorite of the carbonate-uraninite stage is violet turning to nearly black in proximity of uraninite lenses. In both cases, fluorite forms isolated grains, small aggregates or thin veinlets with euhedral crystals in vugs [351]. Galena PbS](https://figures.academia-assets.com/71852809/table_059.jpg)










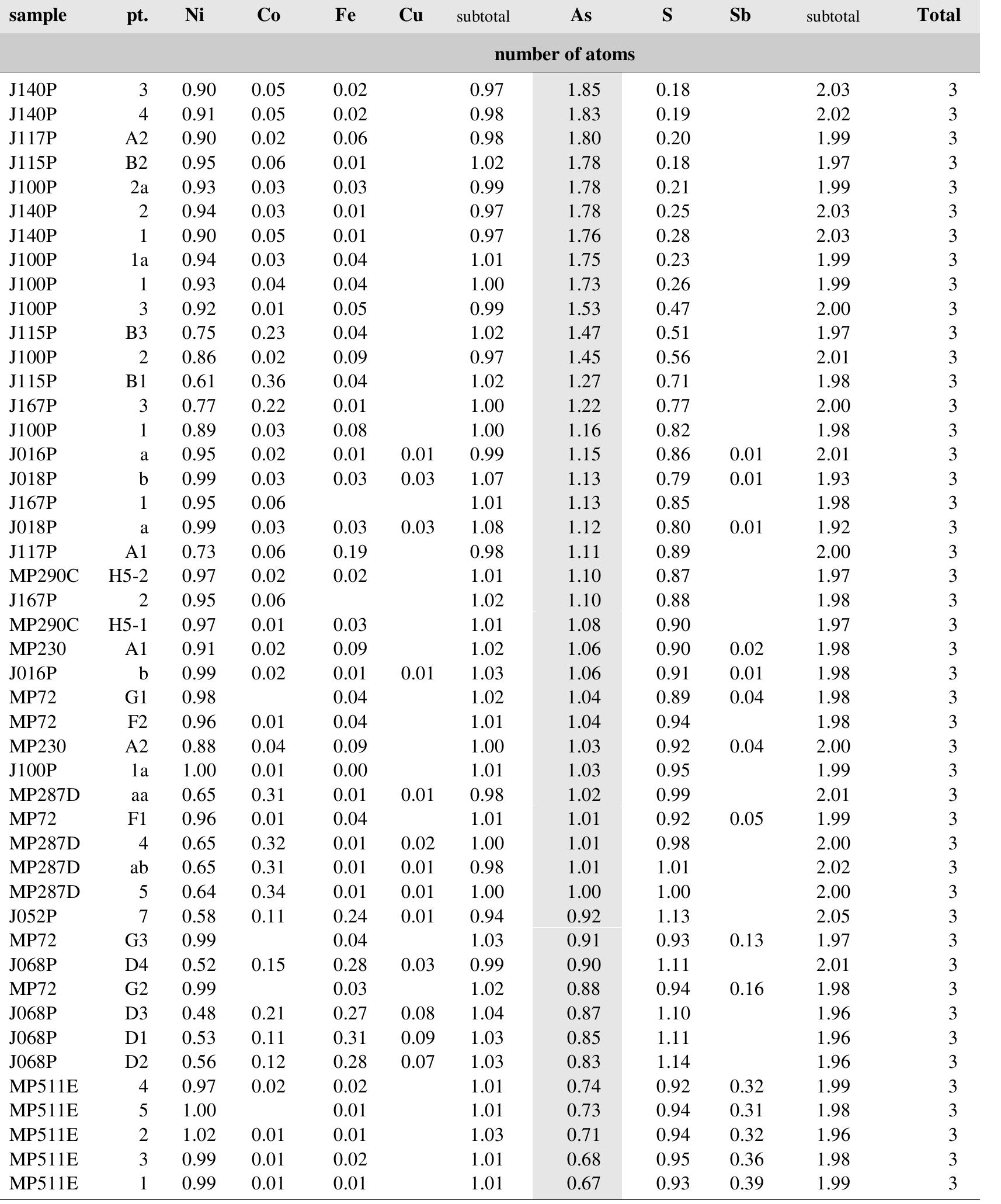






















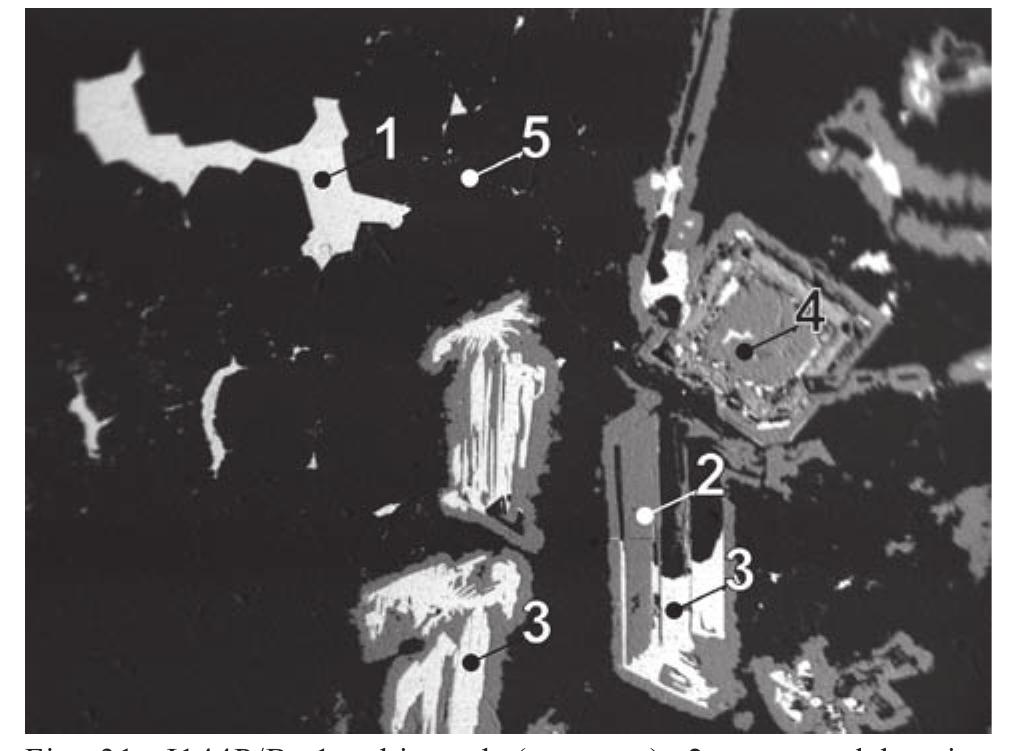
![Fig. 89. JO74P/A-3. 1 — lautite, 2 — léllingite, 3 — bornite, 4 — galena, 5 — quartz. Svornost shaft, Daniel level, intersection of Trojicka vein with Geschieber vein. BSE image. Magnification 320x. A possibility remains that enargite replacing bismuth, reported by Ziikert [423] in a polished section, was also lautite, because of similarity in optical properties of enargite and lautite.](https://figures.academia-assets.com/71852809/figure_091.jpg)












































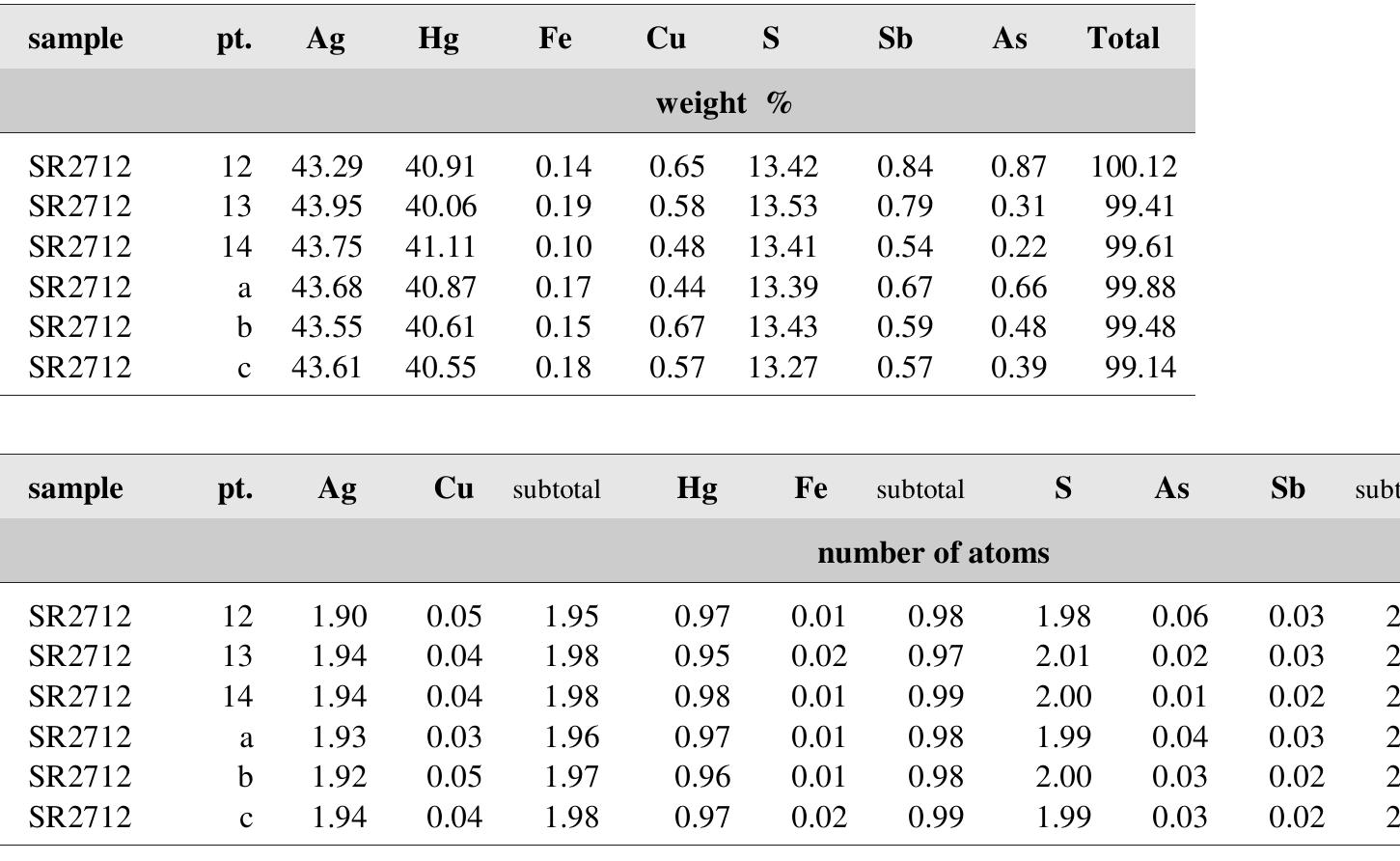
![Table 89. Calculated unit-cell parameters of nickel- skutterudite from Jachymov for the space group /m3. The second type of nickel-skutterudite — cubic crystals or rather their relics partly replaced by bismuth and quartz, and some anhedral crystals, associate not only with bismuth and bismuthinite but also with tennantite, chalcopyrite and younger sulphides. Density corrected for content of bismuth and quartz was 6.23 g/cm? [429]. According to Zepharov- ich [391], density of nickel-skutterudite is 6.89 g/cm’.](https://figures.academia-assets.com/71852809/table_094.jpg)











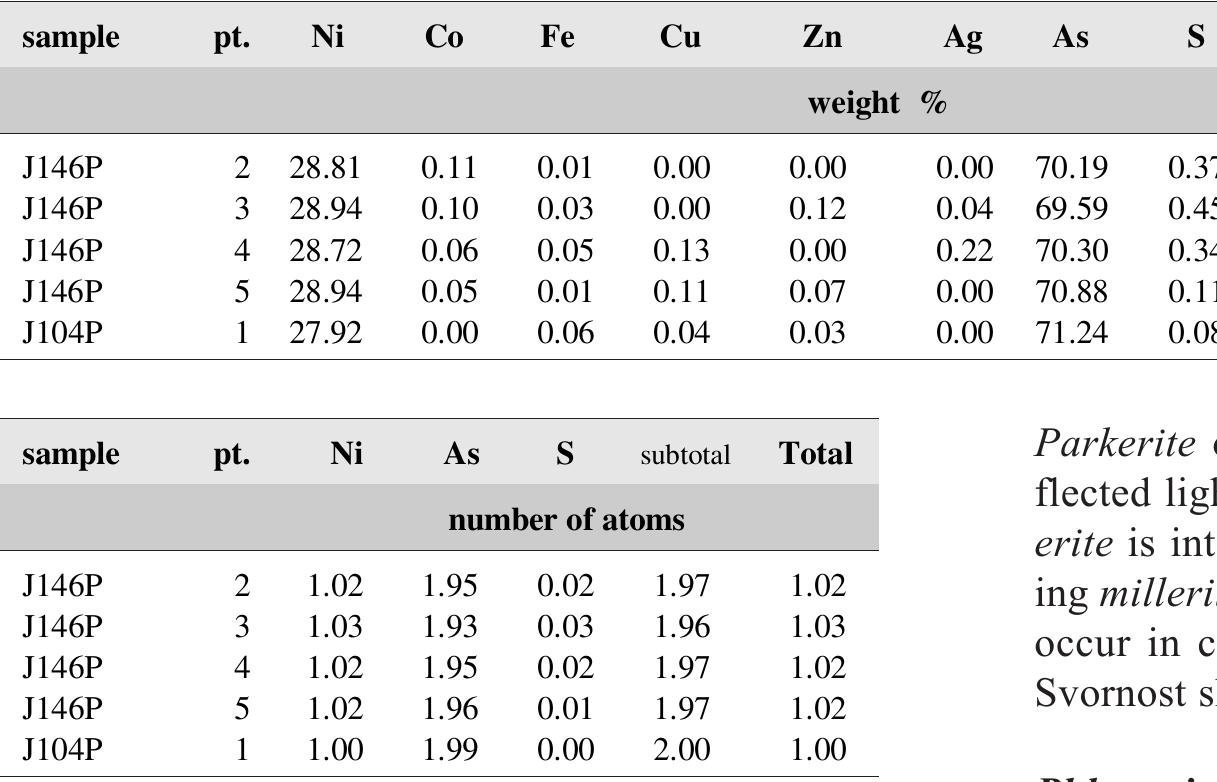














![Table 97. Calculated unit-cell parameters of proustite from Jachymov for the space group R3c. Proustite-pyragyrite minerals close in composition to the end-members occur only exceptionally in the Jachymov ore veins. Pyrargyrite has at least a minor As content, with minimum at 0.2 wt.% As. Both minerals tend to form in- tergrowth in crystallographic orientation and single crys- tals of pure proustite and pyrarargyrite have not been found. Ramdohr [487] described crystallographically ori- entated intergrowth of the two minerals from Andreasberg in Harz, Germany. From Jachymov, Ramdohr mentioned](https://figures.academia-assets.com/71852809/table_104.jpg)




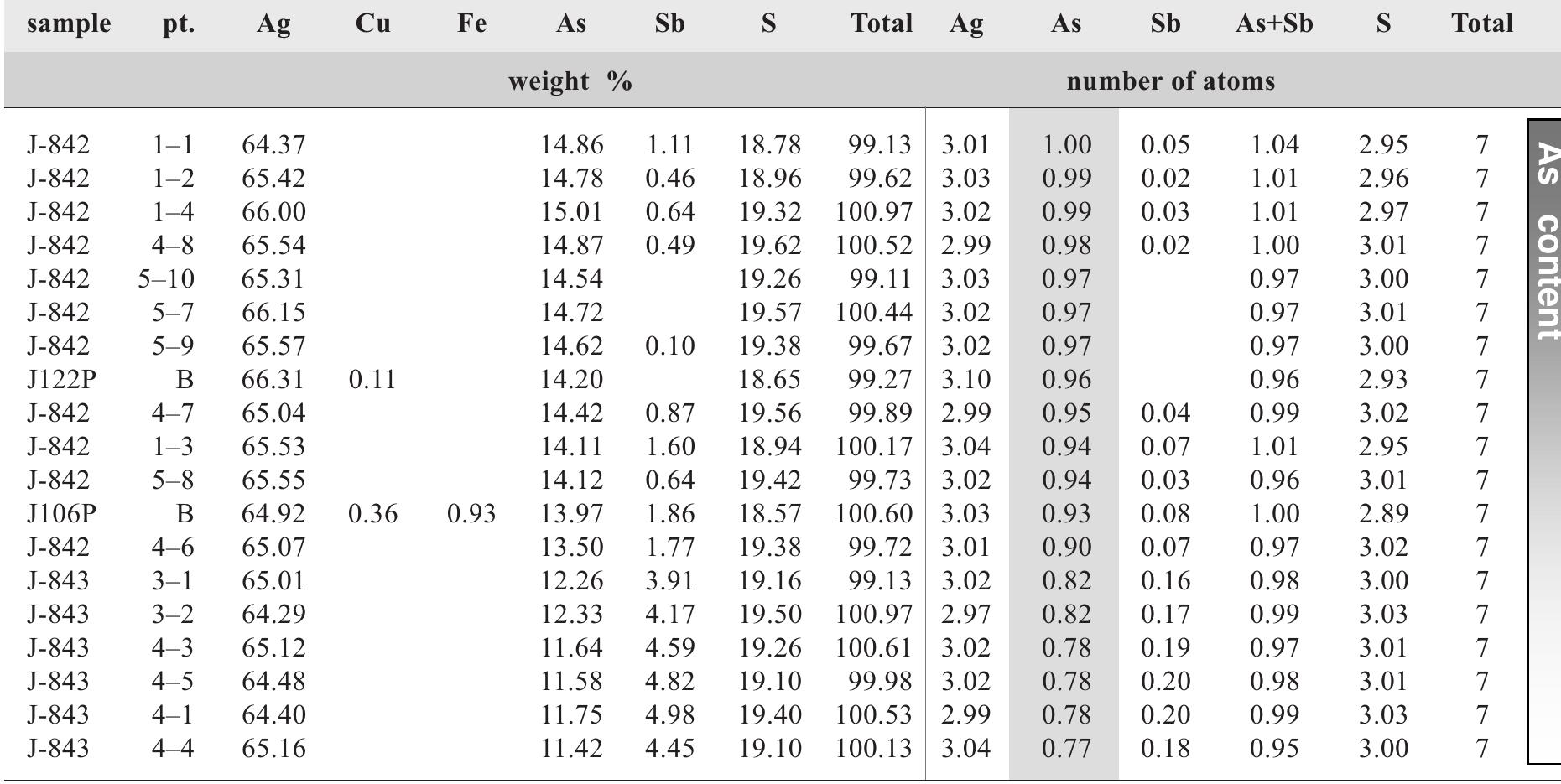












![Fig. 147. Correlation of unit-cell parameter a and As content for pyrites enriched in As from Jachymov. Red symbols show data for As-free pyrite, taken from [499].](https://figures.academia-assets.com/71852809/figure_150.jpg)









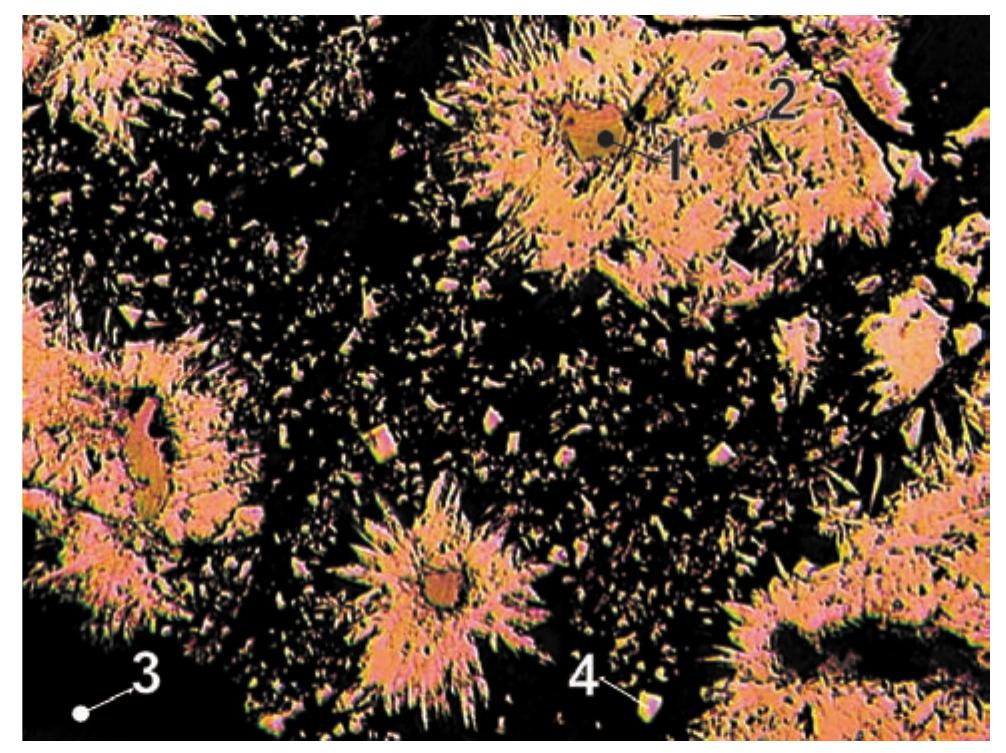










![Table 106. (continued) The outer shape of rammelsbergite aggregates is usu- ally irregular or botryoidal and euhedral crystals are rare. Rammelsbergite rims around native metals show a zon- ing structure (Fig. 150), with individual zones showing different resistence to oxidation in air. The internal struc- ture of zoned aggregates indicates that the zones mimic shape of dendritic silver, serving as matrix for rammels- bergite. It sometimes forms radiating aggregates [351]. The present study confirmed observations by Mrna and Pavlt [351] and characterizes rammelsbergite as belong- ing to abundant minerals in the deposit. It is newly found in rims around bismuth. It also forms individual grains or larger aggregates. It shows a strong anisotropy and ag- gregate extinction in polished sections. It is often asso- ciated with nickel-skutterudite. Reported zoning of ram- melsbergite aggregates may be explained by variation in](https://figures.academia-assets.com/71852809/table_116.jpg)











![Table 113. Calculated unit-cell parameters of W-Sn rutile from Jachymov for the space group P4,/mnm. ization stage, contain rutile up to 100 um long, showing sharply defined growth zones in BSE images. Light zones correspond to rutile with 4-6 wt.% W, 2-2.5 wt.% Fe, 1-1.5 % Sn and dark zones have 0.2-0.7 wt.% W, 0.9-2.4 wt.% Fe, and 0.8-1.4 % Sn. Rocks containing this zoned W-rich rutile carry topaz. A similar W-rich rutile was described from Western Australia [387] both from muscovite schist and quartz vein. It contains 7 wt.% WO,, 2 wt.% Sb,O, and 1 wt.% FeO.](https://figures.academia-assets.com/71852809/table_123.jpg)





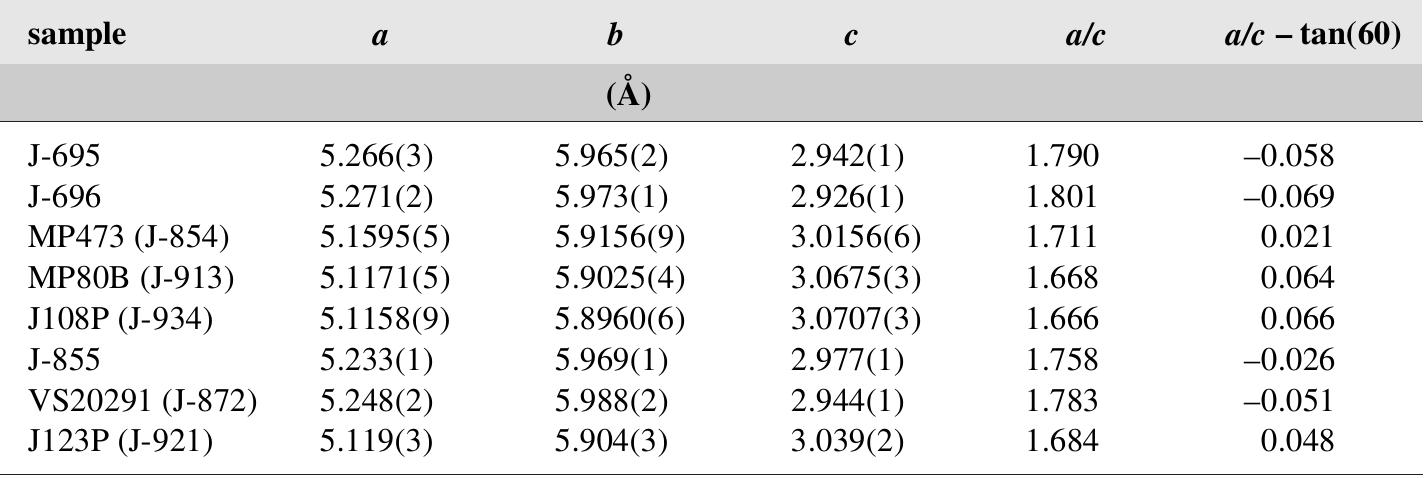































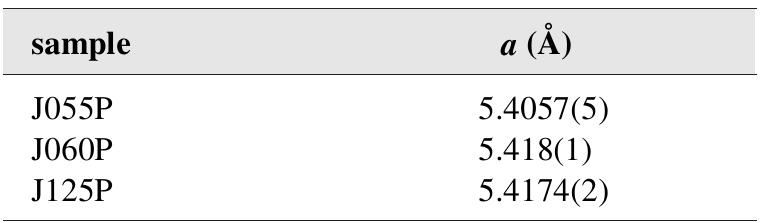





















![Fig. 200. Drawing of sternbergite crystal and twin by Haidinger [492].](https://figures.academia-assets.com/71852809/figure_205.jpg)



![Fig. 202. JO99P/B-1. 1 — stibnite, 2 — pyrite, 3 — calcite. Svornost shaft, Adit level, Hildebrand vein. BSE image. Magnification 16x. Stibnite from Jachymov was described by Ziickert [423]. Mriia and Pavla [351] reported an isolated find of stibnite with pyrite and pyrargyrite from the Svornost shaft, Adit](https://figures.academia-assets.com/71852809/figure_207.jpg)

















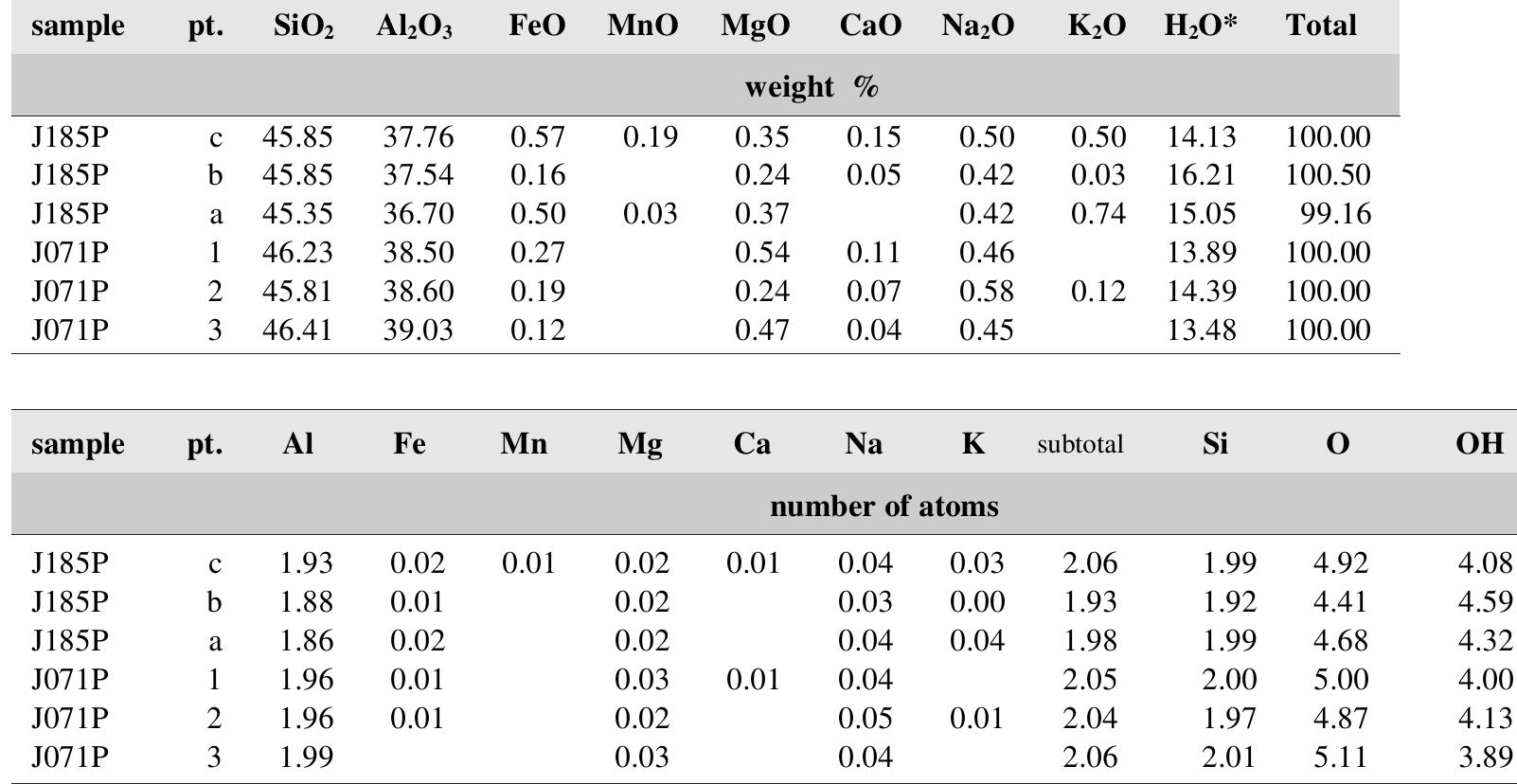
![Vladimir shaft, M-2 vein, contains several tetrahedrite grains enclosed in bornite veinlet. Other well-formed tet- rahedrite crystals come from collection of National Mu- seum, Prague (Fig. 213). neous. This is shown by variation in chemical analyses, particularly in Pb, Zr and Y contents. The increased Si con- tents, interpreted in [150] by entry of Si into interlayer po- sitions along [111], could be alternatively interpreted by a heterogeneous admixture of amorphous gels SiO, - nH,O. The latter interpretation would correspond to geochemi- Tetrahedrite is rare in Jachymov though local tennan- tites contain a common admixture of Sb.](https://figures.academia-assets.com/71852809/table_158.jpg)

![Fig. 213. Well-formed tetrahedrite crystals on calcite (width of figure 1.1 cm). Photo J. & E. Sejkora. Table 143. Calculated unit-cell parameters of tetrahedrite from Jachymov for the space group [43m [443].](https://figures.academia-assets.com/71852809/figure_218.jpg)














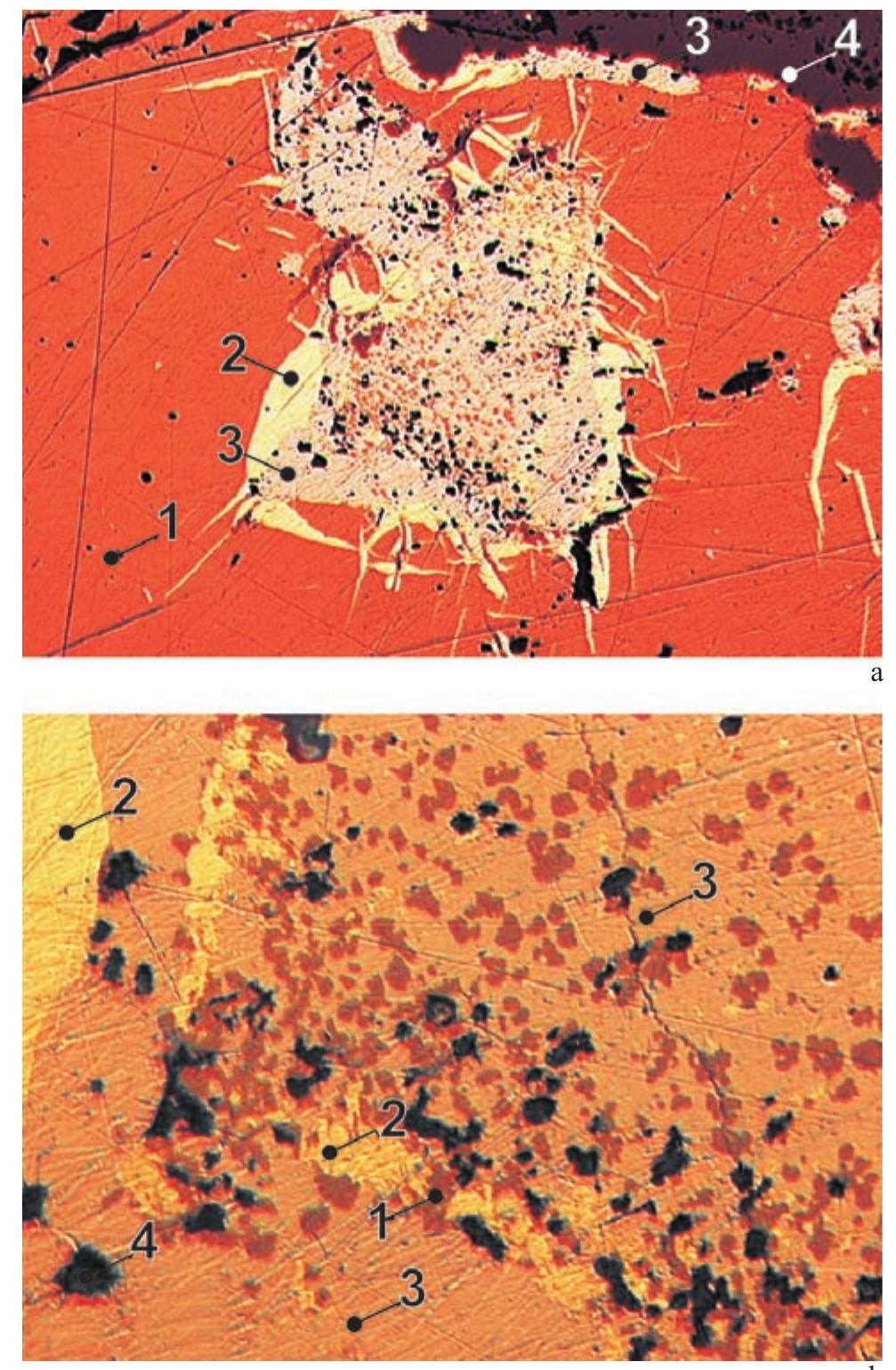















![Fig. 228. VS7651. Crystals of “frieseite”. XRD analysis confirmed that the mineral is a mixture of sternbergite, pyrite and marcasite. Magnification 30x. Photo A. GabaSova. Vrba [532] studied several samples from the Hildebrand and Geister veins with sternbergite and argentopyrite. Two samples representing the two veins contained a min- eral similar to sternbergite but showing different crystal morphology. Vrba introduced the name “frieseite”’ for this](https://figures.academia-assets.com/71852809/figure_234.jpg)





![Fig. 234. Thick tabular singlecrystal of “frieseite” from Jachymov and a twin by Vrba [362]. Crystal forms: c(001), b(010), w(301), 1(102).](https://figures.academia-assets.com/71852809/figure_240.jpg)
![Fig. 235. Orientated intergrowth of a thick tabular crystal of “frieseite” and argentopyrite, by Vrba [362]. Crystal forms: c(001), b(010), q(043), (102), y(101), w(301), t(131).](https://figures.academia-assets.com/71852809/figure_241.jpg)
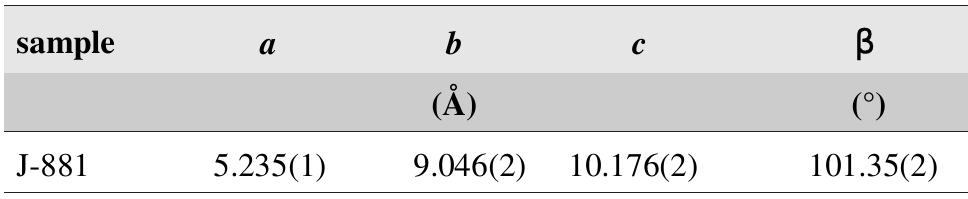
![Fig. 237. Interpretation of Mrna and Pavlu [383] — maucherite rims a fissure in nickeline, arsenide mineralization stage. Eva shaft, vein No. 39. Magnification 80x. Fig. 236. Topotactic replacement of nickeline along small fissures by orientated intergrowths of rammelsbergite (NAP), from [354]. 1 — NAP, 2 — nickeline, 3 — Ni-arsenete? Germany. Reflected light, single polarizer. Magnification 120x.](https://figures.academia-assets.com/71852809/figure_242.jpg)
![No maucherite was identified during the present study, including some original samples. Alteration of nickeline may possibly result in formation of a rare phase NAP (nickeline alteration product), described by Karup-Mgller and Makovicky [354] in an old sample from an unspeci- fied locality in Germany. The phase NAP evolves by par- tial alteration of nickeline along fractures as lamellae 2x20 um in parallel orientation. Border between NAP](https://figures.academia-assets.com/71852809/figure_243.jpg)
Related papers
Journal of Geosciences, 1997
Two hundred and seven secondary mineral species are described and/or referenced. Approximately seventy secondary minerals were known from the district before the present study. All known reliable data on the individual secondary minerals from Jáchymov are presented. New and more complete X-ray powder diffraction data for compreignacite, irhtemite, lavendulan, lindackerite, masuyite, mcnearite, metaschoepite, mottramite, rabbittite, rabejacite, richetite, sodium-zippeite, voglite, zellerite, zippeite, and zýkaite are presented. A list of secondary minerals arranged according to chemical composition and list of "non-secondary" minerals are included at the end of this paper.
Journal of Geosciences, 2003
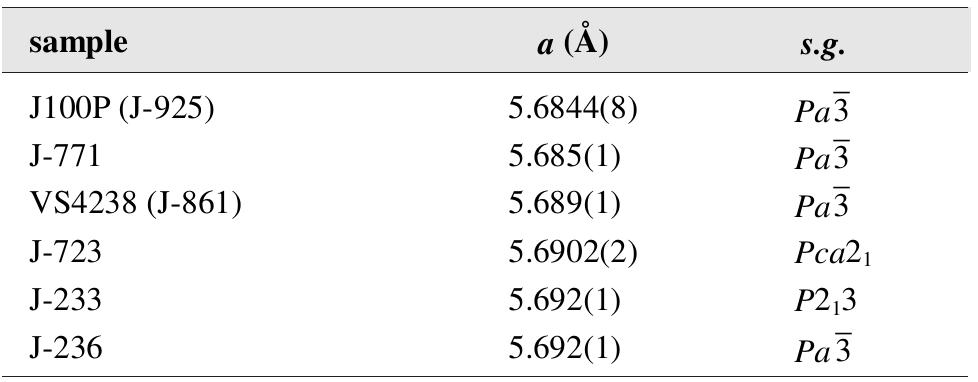
Jáchymov is the type locality for the following primary minerals: argentopyrite, krutovite, millerite, sternbergite, and uraninite. The history of their discovery is described in this contribution. The paper sums up the history of discovery of other type minerals at Jáchymov [560].
Journal of the Czech …, 1997
Jáchymov is type locality for 22 minerals, including 17 secondary minerals. Data on history of discovery and description of new minerals was extracted by search in old literature. Minerals are arranged in the chronological sequence of discovery. Explanation of names of discredited or redefined minerals and some historical names is included at the end of this paper.
Journal of Geosciences, 1997
This paper presents information on relations between physical-chemical properties and genetic features for two hundred and seven secondary minerals and thirty natural phases newly discovered in the Jáchymov ore district. Eight distinct paragenetic groups are described and discussed with respect to formation conditions of the secondary minerals in the Jáchymov ore district.
2003
The paper presents biographic data of persons, after whom new primary minerals discovered at Jáchymov were named (K. Sternberg, W. H. Miller, G. A. Krutov), as well as the recently discovered secondary minerals (J. Vajdák, J. Èejka, J. venek). Biographies of scientists who described new minerals from Jáchymov are given in the following part (F. E. Brückmann, I. Born, A. G. Werner, H. Dauber, G. A. Kenngott, W. Sartorius v. Waltershausen, F. Sandberger, R. Nováèek, R. A. Vinogradova). Biographies of persons who significantly contributed to mineralogy of the Jáchymov ore district are presented in the last section (F. Babánek, J. tìp, R. Zückert, R. P. Dubinkina, R. V. Geceva, F. Mròa, M. Komárek, D. Pavlù). This contribution is a continuation of the 1997 article Who was who? In names of secondary minerals discovered in Jáchymov [561] dealing exclusively with secondary minerals.
Journal of the Czech …, 2003
Ore-forming processes and mineral parageneses of the Jáchymov ore district Rudotvorné procesy a minerální pragenze jáchymovského rudního okrsku
Journal of Geosciences, 1997
Introduction Torbern Olof Bergman was born in Katrineberg, Sweden, the son of Barthold Bergman, sheriff on the royal estate at Katrineberg. Bergman studied mathematics, philosophy, physics and astronomy at the University of Uppsala, graduating in 1756. He later joined the faculty of the University, teaching physics and mathematics, and succeeded to Wallerius chair as Professor of chemistry in 1767. He developed a growing interest in chemistry, mineralogy and crystallography when demonstrated how the stacking of rhombohedral units could produce a scalenohedron. (Haüy later proposed the same thing but denied having known on Bergman's theories.) [217]. Jáchymov is type locality for 17 secondary minerals. Ten of these minerals were named after persons, including some prominent mineralogists of the 19th century. Interesting biographical data on these personalities were assembled during work on the projects of study of the secondary minerals in the Jáchymov ore district (Grant Agency ...
Journal of Geosciences, 1997
This paper describes thirty inorganic compound-secondary mineral phases-found in the nature for the first time. All compounds come from the Jáchymov ore district. All up-to-now available physical and chemical data and references to appropriate literature are given. Crystal structure of phase [ (MoO 2) 2 As 2 O 5 (H 2 O) 2 ]. H 2 O was solved and refined, crystal structures of Ca(H 2 AsO 4) 2 and Mg-villyaellenite were refined by the Rietveld method.
Journal of Geosciences, 2003
Geology and hydrothermal vein system of the Jáchymov (Joachimsthal) ore district Geologie a hydrotermální ilný systém jáchymovského rudního okrsku (26 figs, 1 tab) This contribution provides a brief review of geology of the Kruné hory Mts. (Erzgebirge) and specifically of the Jáchymov (Joachimsthal) deposit. Main types of rocks in the Jáchymov ore district and the role of tectonic processes are described. The position of the ore district is defined much like its tectonic borders. Geological factors controlling the mineralization (especially the so-called five-element mineralization) are summarized. Hydrothermal veins are divided into Morning and Midnight veins according to the classical historical speak of miners, and all vein clusters are listed. Vein hydrothermal five-element mineralization is chronologically divided into separate mineralization stages and their age is estimated.

Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
Related papers
Russian Geology and Geophysics, 2019
Journal of Geosciences, 1997
Review of the Bulgarian Geological Society, 2020
American Mineralogist, 2016
Journal of GEOsciences, 2012
Mineralogical Magazine, 2012
The Canadian Mineralogist, 2016
Geology of Ore Deposits, 2010
 Roman Skala
Roman Skala
